Abstract
All-atom analysis was conducted for cellulose acetate (CA) using molecular dynamics simulation. The intermolecular interactions were elucidated at the amorphous state with degrees of acetyl substitution (DS) of 2, 2.5, and 3, and the energetics of dissolution was treated for H2O, CO2, and CH4. It was observed for the CA amorphous that DS strongly affects the hydrogen bonding among the hydroxy groups of CA and that the short-range packing of pyranose rings becomes tighter with acetylation. The free energy of dissolution was computed by the energy-representation method of solvation. The dissolution into CA was more favorable in the order of H2O > CO2 > CH4, and the DS dependence of the dissolution free energy was evident only for H2O between DS = 2 and 2.5. The roles of the intermolecular interaction components were further addressed. It was seen that the electrostatic component brings the DS dependence of the dissolution free energy for H2O as well as the difference in the affinity to CA between CO2 and CH4. The van der Waals component was still dominant for the nonpolar CO2 and CH4, and the summed contribution to it from the acetyl and main-chain groups of CA was weakly dependent on DS. The connection of the dissolution energetics with the underlying structures is also discussed.
Graphical abstract










Similar content being viewed by others
Data availability
The setup files for MD and free-energy calculation are available at https://doi.org/10.6084/m9.figshare.19534516.v2.
References
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindah E (2015) Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Ban S, Huang C, Yuan XZ, Wang H (2011) Molecular simulation of gas adsorption, diffusion, and permeation in hydrated Nafion membranes. J Phys Chem B 115:11352–11358. https://doi.org/10.1021/jp204141b
Barud HS, de Araújo Júnior AM, Santos DB, de Assunção RMN, Meireles CS, Cerqueira DA, Rodrigues Filho G, Ribeiro CA, Messaddeq Y, Ribeiro SJL (2008) Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim Acta 471:61–69. https://doi.org/10.1016/j.tca.2008.02.009
Basu S, Khan AL, Cano-Odena A, Liu C, Vankelecom IFJ (2010) Membrane-based technologies for biogas separations. Chem Soc Rev 39:750–768. https://doi.org/10.1039/b817050a
Bayly CI, Cieplak P, Cornell WD, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J Phys Chem 97:10269–10280. https://doi.org/10.1021/j100142a004
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Berendsen HJC, Postma JPM, Van Gunsteren WF, Dinola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690. https://doi.org/10.1063/1.448118
Bergenstråhle M, Berglund LA, Mazeau K (2007) Thermal response in crystalline Iβ cellulose: a molecular dynamics study. J Phys Chem B 111:9138–9145. https://doi.org/10.1021/jp072258i
Bialik E, Stenqvist B, Fang Y, Östlund Å, Furó I, Lindman B, Lund M, Bernin D (2016) Ionization of cellobiose in aqueous alkali and the mechanism of cellulose dissolution. J Phys Chem Lett 7:5044–5048. https://doi.org/10.1021/acs.jpclett.6b02346
Bocahut A, Delannoy JY, Vergelati C, Mazeau K (2014) Conformational analysis of cellulose acetate in the dense amorphous state. Cellulose 21:3897–3912. https://doi.org/10.1007/s10570-014-0399-8
Chen GQ, Kanehashi S, Doherty CM, Hill AJ, Kentish SE (2015) Water vapor permeation through cellulose acetate membranes and its impact upon membrane separation performance for natural gas purification. J Memb Sci 487:249–255. https://doi.org/10.1016/j.memsci.2015.03.074
Comell WD, Cieplak P, Bayly CI, Kollman PA (1993) Application of RESP charges to calculate conformational energies, hydrogen bond energies, and free energies of solvation. J Am Chem Soc 115:9620–9631. https://doi.org/10.1021/ja00074a030
de Freitas RRM, Senna AM, Botaro VR (2017) Influence of degree of substitution on thermal dynamic mechanical and physicochemical properties of cellulose acetate. Ind Crops Prod 109:452–458. https://doi.org/10.1016/j.indcrop.2017.08.062
Devanathan R, Venkatnathan A, Dupuis M (2007) Atomistic simulation of nafion membrane: I. Effect of hydration on membrane nanostructure. J Phys Chem B 111:8069–8079. https://doi.org/10.1021/jp0726992
Dou Y, Xu S, Liu X, Han J, Yan H, Wei M, Evans DG, Duan X (2014) Transparent, flexible films based on layered double hydroxide/cellulose acetate with excellent oxygen barrier property. Adv Funct Mater 24:514–521. https://doi.org/10.1002/adfm.201301775
Ehrenstein GW (2001) Polymeric materials: structure, properties, applications. Carl Hanser Verlag, Munich
Elidrissi A, El barkany S, Amhamdi H, Maaroufi A, Hammouti B (2012) New approach to predict the solubility of polymers application: cellulose acetate at various DS, prepared from Alfa “Stipa -tenassicima” of Eastern Morocco. J Mater Environ Sci 3:270–285
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577–8593. https://doi.org/10.1063/1.470117
Frenkel D, Smit B (1996) Understanding molecular simulation: From algorithms to applications, 2nd ed. Academic Press, a division of Harcourt, London, UK
Frisch MJ, Trucks GW, Schlegel HB, et al (2016) G16_a03. Gaussian 16, Revision A.03, Gaussian, Inc., Wallin
Fu Y, Liao L, Lan Y, Yang L, Mei L, Liu Y, Hu S (2012) Molecular dynamics and mesoscopic dynamics simulations for prediction of miscibility in polypropylene/polyamide-11 blends. J Mol Struct 1012:113–118. https://doi.org/10.1016/j.molstruc.2011.12.026
Gallagher JE (2006) Natural gas measurement handbook. Gulf Publishing Company, Houston, Texas
Gartner TE, Jayaraman A (2019) Modeling and simulations of polymers: a roadmap. Macromolecules 52:755–786. https://doi.org/10.1021/acs.macromol.8b01836
General principles of physical testing methods for textiles (2020) Japanese Industrial Standard JIS L0105. Reference no. JIS L 0105: 2020. Japanese Standards Association.
Goga N, Rzepiela AJ, De Vries AH, Marrink SJ, Berendsen HJC (2012) Efficient algorithms for langevin and DPD dynamics. J Chem Theory Comput 8:3637–3649. https://doi.org/10.1021/ct3000876
Hansen JP, McDonald IR (2013) Theory of simple liquids: with applications to soft matter, 4th edn. Academic Press, Oxford
Harris JG, Yung KH (1995) Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J Phys Chem 99:12021–12024. https://doi.org/10.1021/j100031a034
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM (1997) LINCS: A Linear Constraint Solver for molecular simulations. J Comput Chem 18:1463–1472. https://doi.org/10.1002/(SICI)1096-987X(199709)18:12%3c1463::AID-JCC4%3e3.0.CO;2-H
Hofmann D, Fritz L, Ulbrich J, Schepers C, Bhning M (2000) Detailed-atomistic molecular modeling of small molecule diffusion and solution processes in polymeric membrane materials. Macromol Theory Simulations 9:293–327. https://doi.org/10.1002/1521-3919(20000701)9:6%3c293::AID-MATS293%3e3.0.CO;2-1
Ishida T (2020) Theoretical investigation of dissolution and decomposition mechanisms of a cellulose fiber in ionic liquids. J Phys Chem B 124:3090–3102. https://doi.org/10.1021/acs.jpcb.9b11527
Jang SS, Molinero V, Çaǧin T, Goddard WA (2004) Nanophase-segregation and transport in Nafion 117 from molecular dynamics simulations: effect of monomeric sequence. J Phys Chem B 108:3149–3157. https://doi.org/10.1021/jp036842c
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935. https://doi.org/10.1063/1.445869
Kamide K, Saito M (1985) Thermal analysis of cellulose acetate solids with total degrees of substitution of 0.49, 1.75, 2.46, and 2.92. Polym J 17:919–928. https://doi.org/10.1295/polymj.17.919
Kamide K, Okajima K, Kowsaka K, Matsui T (1987) Solubility of cellulose acetate prepared by different methods and its correlationships with average acetyl group distribution on glucopyranose units. Polym J 19:1405–1412. https://doi.org/10.1295/polymj.19.1405
Karayiannis NC, Mavrantzas VG, Theodorou DN (2004) Detailed atomistic simulation of the segmental dynamics and barrier properties of amorphous poly(ethylene terephthalate) and poly(ethylene isophthalate). Macromolecules 37:2978–2995. https://doi.org/10.1021/ma0352577
Kawakami T, Shigemoto I, Matubayasi N (2012) Free-energy analysis of water affinity in polymer studied by atomistic molecular simulation combined with the theory of solutions in the energy representation. J Chem Phys 137:234903. https://doi.org/10.1063/1.4770334
Kawakami T, Shigemoto I, Matubayasi N (2018) Structure and permeability of ionomers studied by atomistic molecular simulation combined with the theory of solutions in the energy representation. J Chem Phys 148:214903. https://doi.org/10.1063/1.5018884
Kinoshita M (2013) A new theoretical approach to biological self-assembly. Biophys Rev 5:283–293. https://doi.org/10.1007/s12551-013-0100-8
Knapp B, Ospina L, Deane CM (2018) Avoiding false positive conclusions in molecular simulation: the importance of replicas. J Chem Theory Comput 14:6127–6138. https://doi.org/10.1021/acs.jctc.8b00391
Kojima H, Handa K, Yamada K, Matubayasi N (2021) Water dissolved in a variety of polymers studied by molecular dynamics simulation and a theory of solutions. J Phys Chem B 125:9357–9371. https://doi.org/10.1021/acs.jpcb.1c04818
Kroon-Batenburg LMJ, Bouma B, Kroon J (1996) Stability of cellulose structures studied by MD simulations. Could mercerized cellulose II be parallel? Macromolecules 29:5695–5699. https://doi.org/10.1021/ma9518058
Kulasinski K, Keten S, Churakov SV, Derome D, Carmeliet J (2014) A comparative molecular dynamics study of crystalline, paracrystalline and amorphous states of cellulose. Cellulose 21:1103–1116. https://doi.org/10.1007/s10570-014-0213-7
Kuo AT, Shinoda W, Okazaki S (2016) Molecular dynamics study of the morphology of hydrated perfluorosulfonic acid polymer membranes. J Phys Chem C 120:25832–25842. https://doi.org/10.1021/acs.jpcc.6b08015
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy into a functional of the electron density formula. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Lindner B, Petridis L, Schulz R, Smith JC (2013) Solvent-driven preferential association of lignin with regions of crystalline cellulose in molecular dynamics simulation. Biomacromol 14:3390–3398. https://doi.org/10.1021/bm400442n
Liu H, Cheng G, Kent M, Stavila V, Simmons BA, Sale KL, Singh S (2012) Simulations reveal conformational changes of methylhydroxyl groups during dissolution of cellulose Iβ in ionic liquid 1-ethyl-3-methylimidazolium acetate. J Phys Chem B 116:8131–8138. https://doi.org/10.1021/jp301673h
Liu S, Hu LF, Zhang WC, Ma HY (2019) Cellulose acetate reverse osmosis membranes for desalination: a short review. Non-Metallic Mater Sci 1:14–24
Mabuchi T, Tokumasu T (2014) Effect of bound state of water on hydronium ion mobility in hydrated Nafion using molecular dynamics simulations. J Chem Phys 141:104904. https://doi.org/10.1063/1.4894813
Manna B, Ghosh A (2019) Dissolution of cellulose in ionic liquid and water mixtures as revealed by molecular dynamics simulations. J Biomol Struct Dyn 37:3987–4005. https://doi.org/10.1080/07391102.2018.1533496
Martinez L, Andrade R, Birgin EG, Martínez JM (2009) PACKMOL: A package for building initial configurations for molecular dynamics simulations. J Comput Chem 30:2157–2164. https://doi.org/10.1002/jcc.21224
Matubayasi N (2019) Energy-representation theory of solutions: its formulation and application to soft, molecular aggregates. Bull Chem Soc Jpn 92:1910–1927. https://doi.org/10.1246/bcsj.20190246
Matubayasi N, Nakahara M (2000) Theory of solutions in the energetic representation. I. Formulation J Chem Phys 113:6070–6081. https://doi.org/10.1063/1.1309013
Matubayasi N, Nakahara M (2002) Theory of solutions in the energy representation. II. Functional for the chemical potential. J Chem Phys 117:3605–3616. https://doi.org/10.1063/1.1495850
Matubayasi N, Nakahara M (2003) Theory of solutions in the energy representation. III. Treatment of the molecular flexibility. J Chem Phys 119:9686–9702. https://doi.org/10.1063/1.1613938
Mori H, Matubayasi N (2018) Resin filling into nano-sized pore on metal surface analyzed by all-atom molecular dynamics simulation over a variety of resin and pore sizes. Polymer (guildf) 150:360–370. https://doi.org/10.1016/j.polymer.2018.06.052
Paajanen A, Vaari J (2017) High-temperature decomposition of the cellulose molecule: a stochastic molecular dynamics study. Cellulose 24:2713–2725. https://doi.org/10.1007/s10570-017-1325-7
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190. https://doi.org/10.1063/1.328693
Pronk S, Páll S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, Van Der Spoel D, Hess B, Lindahl E (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29:845–854. https://doi.org/10.1093/bioinformatics/btt055
Puleo AC, Paul DR, Kelley SS (1989) The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J Memb Sci 47:301–332. https://doi.org/10.1016/S0376-7388(00)83083-5
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23:327–341. https://doi.org/10.1016/0021-9991(77)90098-5
Sakuraba S, Matubayasi N (2014) ERmod: fast and versatile computation software for solvation free energy with approximate theory of solutions. J Comput Chem 35:1592–1608. https://doi.org/10.1002/jcc.23651
Sanaeepur H, Ahmadi R, Sinaei M, Kargari A (2019) Pebax-modified cellulose acetate membrane for CO2/N2 separation. J Membr Sci Res 5:25–32. https://doi.org/10.22079/JMSR.2018.85813.1190
Scholes CA, Kentish SE, Stevens GW (2008) Carbon dioxide separation through polymeric membrane systems for flue gas applications. Recent Patents Chem Eng 1:52–66. https://doi.org/10.2174/2211334710801010052
Scholes CA, Stevens GW, Kentish SE (2012) Membrane gas separation applications in natural gas processing. Fuel 96:15–28. https://doi.org/10.1016/j.fuel.2011.12.074
Silveira RL, Stoyanov SR, Gusarov S, Skaf MS, Kovalenko A (2015) Supramolecular interactions in secondary plant cell walls: Effect of lignin chemical composition revealed with the molecular theory of solvation. J Phys Chem Lett 6:206–211. https://doi.org/10.1021/jz502298q
Stern SA, De Meringo AH (1978) Solubility of carbon dioxide in cellulose acetate at elevated pressures. J Polym Sci Polym Phys Ed 16:735–751. https://doi.org/10.1002/pol.1978.180160415
Stern SA, Kulkarni SS (1982) Solubility of methane in cellulose acetate - conditioning effect of carbon dioxide. J Memb Sci 10:235–251. https://doi.org/10.1016/S0376-7388(00)81412-X
Theodorakis PE, Fytas NG (2012) A study for the static properties of symmetric linear multiblock copolymers under poor solvent conditions. J Chem Phys 136:094902. https://doi.org/10.1063/1.3689303
Uto T, Minamizaki M, Yui T (2020) Molecular dynamics simulation of cellulose I-ethylenediamine complex crystal models. J Phys Chem B 124:134–143. https://doi.org/10.1021/acs.jpcb.9b08153
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general Amber force field. J Comput Chem 25:1157–1174. https://doi.org/10.1002/jcc.20035
Wang W, Li L, ** S, Wang Y, Lan G, Chen Y (2020) Study on cellulose acetate butyrate/plasticizer systems by molecular dynamics simulation and experimental characterization. Polymers (basel) 12:1272. https://doi.org/10.3390/POLYM12061272
Wernersson E, Stenqvist B, Lund M (2015) The mechanism of cellulose solubilization by urea studied by molecular simulation. Cellulose 22:991–1001. https://doi.org/10.1007/s10570-015-0548-8
White LS (2010) Evolution of natural gas treatment with membrane systems. In: Yampolskii Y, Freeman B (eds) Membrane Gas Separation. John Wiley & Sons, pp 313–332
Yamada K, Matubayasi N (2020) Chain-increment method for free-energy computation of a polymer with all-atom molecular simulations. Macromolecules 53:775–788. https://doi.org/10.1021/acs.macromol.9b01952
Yampolskii Y, Pinnau I, Freeman BD (2006) Materials science of membranes for gas and vapor separation. Wiley & Sons, New York
Zugenmaier P (2004) Characterization and physical properties of cellulose acetates. Macromol Symp 208:81–166. https://doi.org/10.1002/masy.200450407
Acknowledgments
We are grateful to Dr. Michinori Yokoi, Dr. Yoshiyuki Yamada, and Mr. Keisuke Towatari of Japan Tobacco Inc., and Dr. Hidekazu Kojima of Osaka University for valuable suggestions. The simulations were performed using OCTOPUS at the Cybermedia Center, Osaka University.
Funding
The authors did not receive support from any organization for the submitted work and have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Simulations were performed by RM. All authors interpreted and analyzed the data. The first draft of the manuscript was written by RM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscription.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
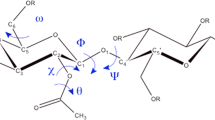
Appendix: Molecular modeling
Appendix: Molecular modeling
The charges on the atomic sites were determined by the procedure of RESP (restrained electrostatic potential) through quantum-chemical calculations by Gaussian 16 (Frisch et al. 2016). In Methods section, we noted that 7 types of monomers were employed with varied degrees and sites of substitution. For each type of the monomers, a homo-tetramer was constructed at n = 4 with two antiparallel units in Fig. 1 and was treated at B3LYP using the 6-31G(d,p) basis set with geometry optimization (Lee et al. 1988; Becke 1993). The partial charges were then determined by RESP for all the atoms and were averaged over equivalent atoms.
To transfer the RESP charges to the MD force field, the following procedures were carried out. For the ether oxygen atoms connecting pyranose rings and those in the methoxy groups at the termini, the charges were averaged over all the ether sites in 7 types of tetramers. A single value of the charge was thus used in MD for the ether oxygen atoms between the pyranose rings and in the terminal methoxy groups. For the inner, two monomers in the tetramer treated by the quantum-chemical calculation, the charges on the same type of atoms were averaged and provided to the corresponding atoms in the inner parts of the CA molecules that were to be simulated with MD. This averaging was done not only for the atoms in the pyranose rings but also for those in the acetyl groups. The charges on the atoms in the terminal monomers and the methyl groups in the methoxy termini were transferred to the corresponding atoms in the termini of the CA molecules, furthermore. The CA molecules were not neutral right after the above and were made neutral by shifting the partial charge on each atom of CA by a uniform constant given by the excess charge divided by the number of atoms in the CA molecule.
Rights and permissions
About this article
Cite this article
Matsuba, R., Kubota, H. & Matubayasi, N. All-atom molecular simulation study of cellulose acetate: amorphous structure and the dissolution of small molecule. Cellulose 29, 5463–5478 (2022). https://doi.org/10.1007/s10570-022-04616-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04616-4