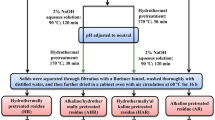
Abstract
Converting food waste, such as garlic straw, into bioethanol offers a promising solution for both food waste management and meeting the increasing energy demands of a growing population. As a low-cost and renewable agro-industrial substrate, garlic straw holds significant potential for bioethanol production. To optimize the enzymatic conversion, pretreatment was performed to facilitate the enzymatic saccharification process by alkaline peroxide and sodium chlorite, resulting in a substrate consisting of 83.07% cellulose, 6.13% hemicelluloses, and 2.09% lignin. The bleached garlic straw (BGS) was hydrolyzed using a cellulolytic complex produced by the hypercellulosic mutant Penicillium occitanis Pol6, aiming to convert cellulose into glucose. The BGS was treated with various enzyme loading (10–50 FPU/g), at different BGS concentration (20–80 g/L) and tween 80 concentration (0–8 g/L) and at different reaction time (24–72 h). The hydrolysis yield from enzymatic saccharification of BGS were evaluated using a Box–Behnken Design. The optimum conditions for the hydrolysis yield were obtained based on surface and contour plots. The maximum predicted hydrolysis yield of 54.08% was obtained as follows: enzyme loading 40 FPU/g, BGS concentration 22 g/L, Tween 80 concentration 6 g/L and hydrolysis time 72 h. Fermentation of hydrolysates of bleached garlic straw (HBGS) carried out using Saccharomyces cerevisiae for 24 h showed that the sugar content decreased over time, while ethanol production increased. Besides, the highest bioethanol production (11.9 g/L) was observed in the 4% HBGS sample after 6 h of alcoholic fermentation. These findings proved the economical production of ethanol using garlic straw as a cheap waste material and also using a low-cost enzymes derivated from filamentous fungi.
Graphical abstract







Similar content being viewed by others
Data availability
No data was used for the research described in the article.
References
Kazemi Shariat Panahi H, Dehhaghi M, Guillemin GJ et al (2022) Bioethanol production from food wastes rich in carbohydrates. Curr Opin Food Sci 43:71–81. https://doi.org/10.1016/j.cofs.2021.11.001
Oberoi HS, Vadlani PV, Saida L et al (2011) Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Manag 31:1576–1584. https://doi.org/10.1016/j.wasman.2011.02.007
Turhan I, Bialka KL, Demirci A, Karhan M (2010) Ethanol production from carob extract by using saccharomyces cerevisiae. Bioresour Technol 101:5290–5296. https://doi.org/10.1016/j.biortech.2010.01.146
Tanaka K, Hilary ZD, Ishizaki A (1999) Investigation of the utility of pineapple juice and pineapple waste material as low-cost substrate for ethanol fermentation by Zymomonas mobilis. J Biosci Bioeng 87:642–646. https://doi.org/10.1016/s1389-1723(99)80128-5
Izmirlioglu G, Demirci A (2015) Enhanced bio-ethanol production from industrial potato waste by statistical medium optimization. Int J Mol Sci 16:24490–24505. https://doi.org/10.3390/ijms161024490
Shahid I, Hussain G, Anis M et al (2023) Enzymatic co-fermentation of onion waste for bioethanol production using saccharomyces cerevisiae and pichia pastoris. Energies 16:2181. https://doi.org/10.3390/en16052181
Verma T, Aggarwal A, Dey P et al (2023) Medicinal and therapeutic properties of garlic, garlic essential oil, and garlic-based snack food: an updated review. Front Nutr. https://doi.org/10.3389/fnut.2023.1120377
Kallel F, Chaabouni Ellouz S (2024) Biovalorization of Garlic Waste to Produce High Value-Added Products. pp 309–332
Rauf A, Abu-Izneid T, Thiruvengadam M et al (2022) Garlic (Allium sativum L.): its chemistry nutritional composition toxicity and anticancer properties. CTMC. https://doi.org/10.2174/1568026621666211105094939
Naureen Z, Bonetti G, Medori MC et al (2022) Foods of the mediterranean diet garlic and mediterranean legumes. J Prev Med Hyg. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2741
Kallel F, Ellouz Chaabouni S (2017) Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: a review. Environ Prog Sustainable Energy 36:1765–1777. https://doi.org/10.1002/ep.12649
Kallel F, Bettaieb F, Khiari R et al (2016) Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind Crops Prod 87:287–296. https://doi.org/10.1016/j.indcrop.2016.04.060
Biely P, Singh S, Puchart V (2016) Towards enzymatic breakdown of complex plant xylan structures: state of the art. Biotechnol Adv 34:1260–1274. https://doi.org/10.1016/j.biotechadv.2016.09.001
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/C5PY00263J
Rabemanolontsoa H, Saka S (2016) Various pretreatments of lignocellulosics. Biores Technol 199:83–91. https://doi.org/10.1016/j.biortech.2015.08.029
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Biores Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
**menes E, Kim Y, Mosier N et al (2011) Deactivation of cellulases by phenols. Enzyme Microb Technol 48:54–60. https://doi.org/10.1016/j.enzmictec.2010.09.006
Wen Z, Liao W, Chen S (2004) Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour Technol 91:31–39. https://doi.org/10.1016/s0960-8524(03)00166-4
Narendra Kumar HK, Chandra Mohana N, Rakshith D et al (2023) Bioethanol production by enzymatic hydrolysis from Aspergillus calidoustus employing different lignocellulosic wastes. Biocatal Agric Biotechnol 53:102847. https://doi.org/10.1016/j.bcab.2023.102847
Qian Y, Liu W, Qu Y, Zhong Y (2019) Enhancement of cellulase production in trichoderma reesei via disruption of multiple protease genes identified by comparative secretomics. Front Microbiol. https://doi.org/10.3389/fmicb.2019.02784
Kaur PP, Arneja JS, Singh J (1998) Enzymic hydrolysis of rice straw by crude cellulase from Trichoderma reesei. Biores Technol 66:267–269. https://doi.org/10.1016/S0960-8524(97)00138-7
Ellouz Chaabouni S, Belguith H, Hassairi I et al (1995) Optimization of cellulase production by penicillium occitanis. Appl Microbiol Biotechnol 43:267–269. https://doi.org/10.1007/BF00172822
Chaabouni SE, Hadj-Taieb N, Mosrati R, Ellouz R (1994) Preliminary assessment of penicillium occitanis cellulase: a further useful system. Enzyme Microb Technol 16:538–542. https://doi.org/10.1016/0141-0229(94)90027-2
Chaabouni SE, Mechichi T, Limam F, Marzouki N (2005) Purification and characterization of two low molecular weight endoglucanases produced by Penicillium occitanis mutant Pol 6. Appl Biochem Biotechnol 125:99–112. https://doi.org/10.1385/abab:125:2:099
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Biores Technol 83:1–11. https://doi.org/10.1016/S0960-8524(01)00212-7
Guan X, Yao H (2008) Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem 106:345–351. https://doi.org/10.1016/j.foodchem.2007.05.041
Bigand V, Pinel C, da Silva PD et al (2013) Influence of liquid or solid phase preparation of cationic hemicelluloses on physical properties of paper. BioResources 8:2118–2134
Jain S, Durand H, Tiraby G (1990) Production of extracellular pectinase enzymes by a mutant (Pol6) of Penicillium occitanis. Appl Microbiol Biotechnol 34:308–312. https://doi.org/10.1007/BF00170048
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475. https://doi.org/10.2307/1266454
Govaerts B (2000) Pharmaceutical experimental design, G. Lewis, D. Mathieu, and R. Phan-Tan-Luu. M. Dekker, New York, 1999, 498 pp., $175. ISBN 0-8247-3860-0. J Chemom 14:93–94. https://doi.org/10.1002/(SICI)1099-128X(200003/04)14:23.3.CO;2-6
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Mathieu D, Nony J, Phan-Tan-Luu R (2000) Nemrod-W Software. LPRAI, Marseille
Wood IP, Elliston A, Ryden P et al (2012) Rapid quantification of reducing sugars in biomass hydrolysates: improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenerg 44:117–121. https://doi.org/10.1016/j.biombioe.2012.05.003
Bouaziz F, Abdeddayem AB, Koubaa M et al (2020) Bioethanol production from date seed cellulosic fraction using saccharomyces cerevisiae. Separations 7:67. https://doi.org/10.3390/separations7040067
Bryan WL (1990) Solid-state fermentation of sugars in sweet sorghum. Enzyme Microb Technol 12:437–442. https://doi.org/10.1016/0141-0229(90)90054-T
Chen Q, Marshall MN, Geib SM et al (2012) Effects of laccase on lignin depolymerization and enzymatic hydrolysis of ensiled corn stover. Bioresour Technol 117:186–192. https://doi.org/10.1016/j.biortech.2012.04.085
Cheng Y-S, Zheng Y, Yu CW et al (2010) Evaluation of high solids alkaline pretreatment of rice straw. Appl Biochem Biotechnol 162:1768–1784. https://doi.org/10.1007/s12010-010-8958-4
Arantes V, Saddler JN (2010) Access to cellulose limits the efficiency of enzymatic hydrolysis: the role of amorphogenesis. Biotechnol Biofuels 3:4. https://doi.org/10.1186/1754-6834-3-4
Xu J, Cheng J, Sharma-Shivappa R, Burns J (2010) Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy Fuels. https://doi.org/10.1021/ef9014718
Kang X, Zhang Y, Li L et al (2020) Enhanced methane production from anaerobic digestion of hybrid Pennisetum by selectively removing lignin with sodium chlorite. Biores Technol 295:122289. https://doi.org/10.1016/j.biortech.2019.122289
Wang Y, Yang Y, Qu Y, Zhang J (2021) Selective removal of lignin with sodium chlorite to improve the quality and antioxidant activity of xylo-oligosaccharides from lignocellulosic biomass. Biores Technol 337:125506. https://doi.org/10.1016/j.biortech.2021.125506
Malik K, Salama E-S, Kim TH, Li X (2020) Enhanced ethanol production by Saccharomyces cerevisiae fermentation post acidic and alkali chemical pretreatments of cotton stalk lignocellulose. Int Biodeterior Biodegrad 147:104869. https://doi.org/10.1016/j.ibiod.2019.104869
Reddy N, Yang Y (2009) Properties and potential applications of natural cellulose fibers from the bark of cotton stalks. Biores Technol 100:3563–3569. https://doi.org/10.1016/j.biortech.2009.02.047
Jones D, Ormondroyd GO, Curling SF, et al (2017) Chemical compositions of natural fibres. In: Advanced High Strength Natural Fibre Composites in Construction. Elsevier, pp 23–58
Zhang YQ, Fu EH, Liang JH (2008) Effect of ultrasonic waves on the saccharification processes of lignocellulose. Chem Eng Technol 31:1510–1515. https://doi.org/10.1002/ceat.200700407
Yu H, Zhang X, Song L et al (2010) Evaluation of white-rot fungi-assisted alkaline/oxidative pretreatment of corn straw undergoing enzymatic hydrolysis by cellulase. J Biosci Bioeng 110:660–664. https://doi.org/10.1016/j.jbiosc.2010.08.002
Zhang J, Tang M, Viikari L (2012) Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour Technol 121:8–12. https://doi.org/10.1016/j.biortech.2012.07.010
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels 4:36. https://doi.org/10.1186/1754-6834-4-36
Sulbarán-de-Ferrer B, Aristiguieta M, Dale BE et al (2003) Enzymatic hydrolysis of ammonia-treated rice straw. Appl Biochem Biotechnol 105–108:155–164. https://doi.org/10.1385/abab:105:1-3:155
Optimization of Multigrain Premix for High Protein and Dietary Fibre Biscuits Using Response Surface Methodology (RSM). https://www.scirp.org/journal/paperinformation?paperid=57089. Accessed 6 Mar 2024
Chen N, Fan J-B, **ang J et al (2006) Enzymatic hydrolysis of microcrystalline cellulose in reverse micelles. Biochim Biophys Acta (BBA) Protein Proteom. https://doi.org/10.1016/j.bbapap.2006.03.015
Qi B, Chen X, Shen F et al (2009) Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind Eng Chem Res 48:7346–7353. https://doi.org/10.1021/ie8016863
Gregg DJ, Saddler JN (1996) Factors affecting cellulose hydrolysis and the potential of enzyme recycle to enhance the efficiency of an integrated wood to ethanol process. Biotechnol Bioeng 51:375–383. https://doi.org/10.1002/(SICI)1097-0290(19960820)51:4%3c375::AID-BIT1%3e3.0.CO;2-F
Liguori R, Soccol CR, de Souza P, Vandenberghe L et al (2015) Second generation ethanol production from brewers’ spent grain. Energies 8:2575–2586. https://doi.org/10.3390/en8042575
Xu Z, Wang Q, Jiang Z et al (2007) Enzymatic hydrolysis of pretreated soybean straw. Biomass Bioenerg 31:162–167. https://doi.org/10.1016/j.biombioe.2006.06.015
Cao W, Sun C, Qiu J et al (2016) Pretreatment of sweet sorghum bagasse by alkaline hydrogen peroxide for enhancing ethanol production. Korean J Chem Eng. https://doi.org/10.1007/s11814-015-0217-5
Fan LT, Lee Y-H, Beardmore DH (1980) Mechanism of the enzymatic hydrolysis of cellulose: effects of major structural features of cellulose on enzymatic hydrolysis. Biotechnol Bioeng 22:177–199. https://doi.org/10.1002/bit.260220113
Wang W, Kang L, Wei H et al (2011) Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Appl Biochem Biotechnol 164:1139–1149. https://doi.org/10.1007/s12010-011-9200-8
Mussatto SI, Dragone G, Fernandes M et al (2008) The effect of agitation speed, enzyme loading and substrate concentration on enzymatic hydrolysis of cellulose from brewer’s spent grain. Cellulose 15:711–721. https://doi.org/10.1007/s10570-008-9215-7
Yáñez R, Alonso JL, Parajó JC (2006) Enzymatic saccharification of hydrogen peroxide-treated solids from hydrothermal processing of rice husks. Process Biochem 41:1244–1252. https://doi.org/10.1016/j.procbio.2005.12.020
Singh A, Bishnoi NR (2012) Optimization of enzymatic hydrolysis of pretreated rice straw and ethanol production. Appl Microbiol Biotechnol 93:1785–1793. https://doi.org/10.1007/s00253-012-3870-1
Sui W, Liu X, Sun H et al (2021) Improved high-solid loading enzymatic hydrolysis of steam exploded corn stalk using rapid room temperature γ-valerolactone delignification. Ind Crops Prod 165:113389. https://doi.org/10.1016/j.indcrop.2021.113389
Zain MM, Mohammad AW, Harun S et al (2018) Synergistic effects on process parameters to enhance enzymatic hydrolysis of alkaline oil palm fronds. Ind Crops Prod 122:617–626. https://doi.org/10.1016/j.indcrop.2018.06.037
Vlasenko EYu, Ding H, Labavitch JM, Shoemaker SP (1997) Enzymatic hydrolysis of pretreated rice straw. Biores Technol 59:109–119. https://doi.org/10.1016/S0960-8524(96)00169-1
Guo J, Li Z, Su L et al (2018) Optimization of acid pretreatment and enzymatic hydrolysis on the production of ethanol fuel from waste banana peels. Energy & Environment 29:1354–1364
Devi A, Bajar S, Sihag P et al (2023) A panoramic view of technological landscape for bioethanol production from various generations of feedstocks. Bioengineered 14:81–112. https://doi.org/10.1080/21655979.2022.2095702
Ask M, Olofsson K, Felice T et al (2012) Challenges in enzymatic hydrolysis and fermentation of pretreated arundo donax revealed by a comparison between SHF and SSF. Process Biochem 47:1452–1459. https://doi.org/10.1016/j.procbio.2012.05.016
Scordia D, Cosentino S, Lee J-W, Jeffries T (2011) Dilute oxalic acid pretreatment for biorefining giant reed (Arundo donax L.). Biomass Bioenerg 35:3018–3024. https://doi.org/10.1016/j.biombioe.2011.03.046
Khawla BJ, Sameh M, Imen G et al (2014) Potato peel as feedstock for bioethanol production: a comparison of acidic and enzymatic hydrolysis. Ind Crops Prod 52:144–149. https://doi.org/10.1016/j.indcrop.2013.10.025
Separations | Free Full-Text | Bioethanol Production from Date Seed Cellulosic Fraction Using Saccharomyces cerevisiae. https://www.mdpi.com/2297-8739/7/4/67. Accessed 26 Feb 2024
Arapoglou D, Varzakas T, Vlyssides Α, Israilides C (2010) Ethanol production from potato peel waste (PPW). Waste manag 30:1898–1902. https://doi.org/10.1016/j.wasman.2010.04.017
Keshav PK, Shaik N, Koti S, Linga VR (2016) Bioconversion of alkali delignified cotton stalk using two-stage dilute acid hydrolysis and fermentation of detoxified hydrolysate into ethanol. Ind Crops Prod 91:323–331. https://doi.org/10.1016/j.indcrop.2016.07.031
Chaudhary A, Akram AM, Aihetasham A et al (2021) Punica granatum waste to ethanol valorisation employing optimized levels of saccharification and fermentation. Saudi J Biol Sci 28:3710–3719. https://doi.org/10.1016/j.sjbs.2021.04.049
Funding
This work was supported financially by the Ministry of Higher Education and Scientific Research of Tunisia through a grant to “Laboratory of Plant Improvement and Valorization of Agricultural Resources-ENIS.”
Author information
Authors and Affiliations
Contributions
We, all authors, certified that we have contributed to the work described in the manuscript. By common consent, we agree to submit it for publication in your esteemed journal. We also state, on our honor, that the content of this manuscript has not been published nor has it been submitted elsewhere.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kallel, F., Neifar, M., Kacem, I. et al. Optimization of enzymatic hydrolysis and fermentation of bleached garlic straw for bioethanol production. J Mater Cycles Waste Manag (2024). https://doi.org/10.1007/s10163-024-02016-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10163-024-02016-3