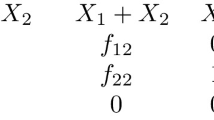Abstract
We address several questions in reduced versus extended networks via the elimination or addition of intermediate complexes in the framework of chemical reaction networks with mass-action kinetics. We clarify and extend advances in the literature concerning multistationarity in this context, mainly from Feliu and Wiuf (J R Soc Interface 10:20130484, 2013), Sadeghimanesh and Feliu (Bull Math Biol 81:2428–2462, 2019), Pérez Millán and Dickenstein (SIAM J Appl Dyn Syst 17(2):1650–1682, 2018), Dickenstein et al. (Bull Math Biol 81:1527–1581, 2019). We establish general results about MESSI systems, which we use to compute the circuits of multistationarity for significant biochemical networks.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
All Maple source code used here is available at https://archive.softwareheritage.org/browse/origin/directory/?origin_url=https://github.com/RickRischter/Multistationarity-BioNetworks.
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, 4th edn. Garland Science, New York
Balaji R, Bapat RB, Goel S (2020) Resistance distance in directed cactus graphs. Electron J Linear Algebra 36:277–292. https://doi.org/10.13001/ela.2020.5093
Banaji M (2023) Splitting reactions preserves nondegenerate behaviors in chemical reaction networks. SIAM J Appl Math 83(2):748–769
Banaji M, Boros B, Hofbauer J (2022) Adding species to chemical reaction networks: preserving rank preserves nondegenerate behaviours. Appl Math Comput 426:127109
Banaji M, Pantea C (2018) The inheritance of nondegenerate multistationarity in chemical reaction networks. SIAM J Appl Math 78(2):1105–1130
Conradi C, Feliu E, Mincheva M, Wiuf C (2017) Identifying parameter regions for multistationarity. PLoS Comput Biol 13(10):e1005751
Conradi C, Flockerzi D, Raisch J (2008) Multistationarity in the activation of a MAPK: parametrizing the relevant region in parameter space. Math Biosci 211(1):105–131
Conradi C, Kahle T (2015) Detecting binomiality. Adv Appl Math 71:52–67
Dessauges C, Mikelson J, Dobrzyński M, Jacques M-A, Frismantiene A, Gagliardi PA, Khammash M, Pertz O (2022) Optogenetic actuator—ERK biosensor circuits identify MAPK network nodes that shape ERK dynamics. Mol Syst Biol 18(6):e10670
Dickenstein A (2016) Biochemical reaction networks: an invitation for algebraic geometers. MCA 2013, Contemp Math 656:65–83
Dickenstein A (2020) Algebraic geometry tools in systems biology. Notices AMS 67(11):1706–1715
Dickenstein A, Pérez Millán M, Shiu A, Tang X (2019) Mutistationarity in structured reaction networks. Bull Math Biol 81:1527–1581
Feliu E, Wiuf C (2012) Enzyme-sharing as a cause of multi-stationarity in signalling systems. J R Soc Interface 9(71):1224–1232
Feliu E, Wiuf C (2013) Simplifying biochemical models with intermediate species. J R Soc Interface 10:20130484
Fritsche-Guenther R, Witzel F, Sieber A, Herr R, Schmidt N, Braun S, Brummer T, Sers C, Nils Blüuthgen N (2011) Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol Syst Biol 7:489
Giaroli M, Rischter R, Pérez Millán M, Dickenstein A (2019) Parameter regions that give rise to 2[n/2] +1 positive steady states in the n-site phosphorylation system. Math Biosci Eng 16:7589–7615
Gunawardena J (2007) Distributivity and processivity in multisite phosphorylation can be distinguished through steady-state invariants. Biophys J 93:3828–3834
Hou Y, Chen J (2015) Inverse of the distance matrix of a cactoid digraph. Linear Algebra Appl 475:1–10
Huang C-YF, Ferrell JE (1996) Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 93(19):10078–10083
Joshi B, Shiu A (2013) Atoms of multistationarity in chemical reaction networks. J Math Chem 51:153–178
Kolch W, Halasz M, Granovskaya M, Kholodenko B (2015) The dynamic control of signal transduction networks in cancer cells. Nat Rev Cancer 15:515–527
Maplesoft (2014) Maple 18. Maplesoft, a division of Waterloo Maple Inc., Waterloo, Ontario
Markevich N, Hoek J, Kholodenko B (2004) Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J Cell Biol 164(3):353–359
Mirzaev I, Gunawardena J (2013) Laplacian dynamics on general graphs. Bull Math Biol 75(11):2118–49
Müller S, Feliu E, Regensburger G, Conradi C, Shiu A, Dickenstein A (2016) Sign conditions for injectivity of generalized polynomial maps with applications to chemical reaction networks and real algebraic geometry. Found Comput Math 16(1):69–97
Nam K-M, Gyori BM, Amethyst SV, Bates DJ, Gunawardena J (2020) Robustness and parameter geography in post-translational modification systems. PLoS Comput Biol 16:e1007573
Patel A, Shvartsman S (2018) Outstanding questions in developmental ERK signaling. Development 145(14):dev143818
Pérez Millán M, Dickenstein A (2018) The structure of MESSI biological systems. SIAM J Appl Dyn Syst 17(2):1650–1682
Pérez Millán M, Shiu A, Conradi C, Dickenstein A (2012) Chemical reaction systems with toric steady states. Bull Math Biol 74(5):1027–1065
Sadeghimanesh A, Feliu E (2019) The multistationarity structure of networks with intermediates and a binomial core network. Bull Math Biol 81:2428–2462
Suwanmajo T, Krishnan J (2015) Mixed mechanisms of multi-site phosphorylation. J R Soc Interface 12(107):20141405
Tutte WT (1948) The dissection of equilateral triangles into equilateral triangles. Proc Camb Philos Soc 44:463–482
Acknowledgements
We are very thankful to Eugenia Ellis and Andrea Solotar for organizing the excellent project “Matemáticas en el Cono Sur”, which lead to this work. We also thank Eugenia Ellis for the warm hospitality in Montevideo, Uruguay, on December 3–7, 2018, where we devised our main results.
Funding
Rick Richter was partially supported by FAPEMIG RED-00133-21. AD, MG and MPM were partially supported by UBACYT 20020220200166BA and CONICET PIP 11220200100182CO, Argentina. We also acknowledge partial support from the PUE grant 22920170100037 of the Instituto de Investigaciones Matemáticas Luis A. Santaló, that covered a visit of Rick Rischter to Buenos Aires to end this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no Conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Proofs of our main results
Proofs of our main results
In this section we give the proofs of Theorems 2.15, 2.16, 5.2, 6.6 and 6.7. We also include the proof of Lemma 6.9.
Proof of Theorem 2.15
The proof is an extension of that in Theorem 5.1 in Feliu and Wiuf (2013). The species of G are ordered as: \(X_1,\ldots , X_n, U_1,\ldots , U_p\), and the species of \(G_{red}\) are \(X_1,\ldots , X_n\). Given reaction rate constants \(\kappa =(\kappa _{yy'})\) and \(\theta \in {\mathbb {R}}_{>0}\), they define a curve of rate constants \(\kappa ^{\theta }=(\kappa ^{\theta }_{yy'})\) by \(\kappa ^{\theta }_{yy'}= \frac{\kappa _{yy'}}{\theta }\) if y is an intermediate species and \(\kappa ^{\theta }_{yy'}=\kappa _{yy'}\) otherwise. Then it can be checked that for any \(\kappa \in {\mathcal {F}}_{\tau ^0}\), \(\kappa ^{\theta }\in {\mathcal {F}}_{\tau ^0}\) and \(\mu ^{\theta }_{i,y}=\theta \, \mu _{i,y}\). Therefore, given any positive \(\varepsilon \), if we take \(\theta \) small enough it happens that \(\kappa ^{\theta }\in {\mathcal {F}}_{\tau ^0, \varepsilon }\), and then, \({\mathcal {F}}_{\tau ^0,\varepsilon }\) is a nonempty open set of \({\mathcal {F}}_{\tau ^0}\).
Let now \(g_1, \ldots , g_n\) be the polynomials defined in (2.4) associated to the network \(G_{red}\). As there exists a non-degenerate positive steady state of \(G_{red}\) in the stoichiometric compatibility class \({\mathcal {S}}_c\), without loss of generality we can assume by Lemma 2.14 that \(g_{d+1}, \ldots , g_n\) are linearly independent and generate the \({\mathbb {R}}\)-vector space generated by all the \(g_i\)’s. It follows that \(f_{d+1}, \ldots , f_n\) are also linearly independent and generate all the \(f_i\)’s. As we want to prove that there exist \(\kappa \) such that the system \({\bar{\ell }}_1(x,u) - c_1 =\cdots ={\bar{\ell }}_d(x,u) - c_d= f_{\kappa ,1}(x,u)=\cdots =f_{\kappa ,n+p}(x,u)=0\) has m positive non-degenerate solutions, we can consider the solutions of the equivalent system defined by
where \(f_{\kappa ,i}(x,u)\) is the i-th coordinate of the function \(f_{\kappa }(x,u)\) as in (1.1). Then, a vector (x, u) is a steady state of G for the reaction rate constants \(\kappa \) and for the stoichiometric compatibility class defined by \(c_1,\ldots ,c_d\), if and only if \(f_{c,\kappa }(x,u)=0\).
Analogously, we consider the system augmented by conservation laws corresponding to the network \(G_{red}\). A vector x is a steady state of \(G_{red}\) for reaction rate constants \(\tau \) in the stoichiometric compatibility class defined by \(c_1,\ldots ,c_d\) if and only if x is a zero of the following function:
where
In the proof of Theorem 5.1 in Feliu and Wiuf (2013) and recalled in Lemma 2.8, it is shown that the system in (A.1) can be transformed via linear operations into the equations below. That is, there exists an invertible matrix \(K^*\) such that:
where \(\varphi _i(x) =\sum _{y\in {{\mathscr {C}}_{G_{red}}}}{\mu _{i,y}(\kappa ) x^y}\) for \(i=1, \ldots , p\), \(\varphi = (\varphi _1, \ldots , \varphi _p)\), as in (2.4), and \(a_{ik}=\ell _i(y^{(k)})\) with \(y^{(k)}\) a core complex in the same connected component as \(U_k\). Then, the Jacobian matrix of \({f}_{c,\kappa }\) evaluated at (x, u) is nonsingular if and only if the Jacobian matrix of the right-hand side equations evaluated at (x, u) is nonsingular.
Note also that, if \(T(\kappa )=\tau \), then \(f_{\kappa ,d+1}(x,\varphi (x))=g_{\tau ,d+1}(x),\ldots ,f_{\kappa ,n}(x,\varphi (x))=g_{\tau ,n}(x)\). Take now all the nonzero coefficients \(\mu _{i,y}\), for all \(i=1,\ldots ,p\) and all complexes \(y\in G_{red}\), and let N be the number of nonzero \(\mu _{i,y}\) coefficients. Let \(\mu \in {\mathbb {R}}_{>0}^N\) be the vector with coordinates \(\mu _{i,y}\), in some order. Fix \(c_1,\ldots ,c_d\), \(\tau ^0 \in \textrm{im}(T)\) and consider the function \(F_{c,\tau ^0}:{\mathbb {R}}^n\times {\mathbb {R}}^p \times {\mathbb {R}}^N \rightarrow {\mathbb {R}}^{n+p}\) defined by:
Assume now that \(G_{red}\) has m nondegenerate positive steady states \(x^{(i)}\in {\mathbb {R}}^n_{>0}\), \(i=1,\ldots ,m\) in the stoichiometric compatibility class defined by the total amounts \(c_1,\ldots ,c_d\), and for the reaction rate constants \(\tau ^0\).
For \(\mu =0\), \(F_{c,\tau ^0}(x^{(i)},0,0)=0\), because \(\ell _j(x^{(i)})=c_j\) for \(j=1,\ldots ,d\) and \(g_{\tau ^0,j}(x^{(i)})=0\) for all \(j=d+1,\ldots ,m\), and the Jacobian matrix of \(F_{c,\tau ^0}(x,u,0)\) has the form:
Since the steady states \(x^{(i)}\) are nondegenerate, \(J_x(g_{c,\tau ^0})\) evaluated at \(x^{(i)}\) is nonsingular for each \(i=1,\ldots ,m\). Then, \(J_{(x,u)}(F_{c,\tau ^0})\) evaluated at \((x^{(i)},0,0)\) is nonsingular for each \(i=1,\ldots ,m\). By the Implicit Function Theorem applied to F at \((x^{(i)},0,0)\), there exists an open set \({\mathcal {U}}_i\subset {\mathbb {R}}^N\), with \(0 \in {\mathcal {U}}_i\), and an open set \({\mathcal {V}}_i\in {\mathbb {R}}^n\times {\mathbb {R}}^p\), with \((x^{(i)},0)\in {\mathcal {V}}_i\) such that for all \(\mu \in {\mathcal {U}}_i\), there is a vector \((x^{(i)}(\mu ),u^{(i)}(\mu ))\in {\mathcal {V}}_i\) such that \(F_{c,\tau ^0}(x^{(i)}(\mu ),u^{(i)}(\mu ),\mu )=0\).
Because \(x^{(i)}>0\) and \(x^{(i)}\) is a nondegenerate steady state, we can take the open set \({\mathcal {U}}_i\) such that \(x^{(i)}(\mu )>0\) and \(J_{(x,u)}(F_{c,\tau ^0})(x^{(i)}(\mu ),u^{(i)}(\mu ),\mu )\) is nonsingular for all \(\mu \in {\mathcal {U}}_i\). We take \({\mathcal {U}}_i^+={\mathcal {U}}_i\cap {\mathbb {R}}^N_{> 0}\). Since \(x^{(i)}(\mu )>0\), it follows that \(u^{(i)}(\mu )>0\) for all \(\mu \in {\mathcal {U}}_i^+\), by construction. Because the \(x^{(i)}\) are distinct, we can moreover choose the open sets \({\mathcal {U}}_i\) (smaller if needed, contained in the original \({\mathcal {U}}_i\)) such that \(\cap _{i=1}^m {\mathcal {V}}_i=\emptyset \).
Now we take \({\mathcal {U}}=\cap _{i=1}^m {\mathcal {U}}_i^+\). If \(\kappa >0\) is such that \(T(\kappa )=\tau ^0\), and \(\mu =\mu (\kappa )\) as in (2.6) is such that \(\mu \in {\mathcal {U}}\), then the original network G has m nondegenerate positive steady states \((x^{(i)}(\mu ),u^{(i)}(\mu ))\), \(i=1,\ldots ,m\) in the stoichiometric compatibility class defined by \(c_1,\ldots ,c_d\). \(\square \)
Proof of Theorem 2.16
If G is an extension network obtained by adding basic extensions, by Remark 2.11 we know that \(u_i=\mu _ix^{y^i}\), where in the first two cases we have:

In the first type of extension, given any choice of positive rate constants \(\kappa _{U_iy}, \kappa _{U_iy'}\) if we pick \(\kappa _{yU_i}=\tau _{yy'}\frac{\kappa _{U_iy}+\kappa _{U_iy'}}{\kappa _{U_iy'}}\) then \(T_{yy'}(\kappa ) = \tau _{yy'}\). For the second type of extension we can also solve for \(\kappa _{yU_i}\) for any choice of \(\kappa _{U_iy'}\). In the case of a canonical extension,  we have that \({{\mathcal {F}}}_{\tau ^0}\) is the whole set of parameters \(\kappa \) because \(y =y'\).
we have that \({{\mathcal {F}}}_{\tau ^0}\) is the whole set of parameters \(\kappa \) because \(y =y'\).
Consider \(\varepsilon _0\) as in the statement of Theorem 2.15. It is clear that there exists \(M_0 >0\) such that if \(\kappa _{U_iy'}>M_0\) then \(\mu _i<\varepsilon _0\) for every i, and the result follows by Theorem 2.15. \(\square \)
Proof of Theorem 5.2
We apply Theorem 5.3. Suppose that we add one intermediate \(U_{\ell _0}\) in the canonical form:
By Theorem 6.6, this new network is lebn and we can therefore parametrize the positive steady states by monomials. Call with small letters the concentrations of the species. We can write the concentration of all the species at steady state in terms of the concentrations \(s_0\), e, and f in the following way:
where \(\psi _{\ell }(\kappa )\) and \(\phi (\kappa )\) are rational functions of the rate constants \(\kappa \) which are defined in the positive orthant.
We consider the matrix A of exponents of the monomials in the parametrization (A.5) with the following order of the species: \(S_0=S_{i_0}\), \(S_1\), \(\ldots \), \(S_n=S_{i_k}\), E, F and \(U_{\ell _0}\).

We also consider the matrix W of conservation relations:

If we take the submatrices of W and A with columns corresponding to the species \(S_0=S_{i_0}\), E and F, the corresponding determinants have the same sign. But if we take the submatrices of W and A with columns corresponding to the species \(S_n=S_{i_k}\), F and \(Y_{\ell _0}\), the determinants have opposite sign, and then the network is multistationary.
When we add one intermediate \(U_j\) in the canonical form
the network is also multistationary and the proof is analogous to the previous one. In this case, we can obtain a parametrization of the concentration of the species at steady state similar as in (A.5) in terms of \(s_0\), e and f. We have the corresponding matrix of monomials:

and the matrix of conservation relations:

As before, if we take the submatrices of W and A with columns corresponding to the species \(S_0=S_{i_0}\), E and F, the corresponding determinants have the same sign. But if we take the submatrices of W and A with columns corresponding to the species \(S_0=S_{i_{i_0}}\), E and \(U_{j}\), the corresponding determinants have opposite sign, and then the network is multistationary.
Now, it remains to check that if we add the all the intermediates \(U_{\ell }\) for each \(i_{k-1}\le \ell < i_{k}=n\) and the intermediate \(U_{i_1}\) in the canonical form, the network is monostationary. That is, if we add simultaneously all the intermediates in the following form:
the network is monostationary. In this case, any of its subnetworks is also monostationary and we are done. We can also express all the concentrations of the species in terms of \(s_0\), e, and f, in a similar way as in parametrization (A.5). Then, we take the corresponding matrices A and W with the following order of species: \(S_0=S_{i_0}\), \(S_1\), \(\ldots \), \(S_n=S_{i_k}\), E, F and \(Y_{i_{k-1}}\), \(\ldots \), \(Y_{i_k-1}\) and \(U_{i_1}\):


Note that the first columns corresponding to the substrates \(S_{\ell }\) are the same as in the matrices of the previous cases. Once again, if we take the submatrices of W and A with columns corresponding to the species \(S_0=S_{i_0}\), E and F, the corresponding determinants are equal to one. Now we have to check that \(\det (W_J)\det (A_J)\ge 0,\) for all subset J, with \(|J|=3\) and we can conclude that the network is monostationary.
Since A and W have several repeated columns it is enough to check that for all integers j with \(0\le j \le k\) and for all subsets J with \(|J|=3\) we have \(\det (W'_J)\det (A'_J)\ge 0,\) where
This can be easily done in a Computer Algebra System or even by hand. \(\square \)
Proof of Lemma 6.9
Consider a leaf of the tree representation \(T_{{\widetilde{G}}}\) of \({\widetilde{G}}\) (that is, a vertex of degree one). This leaf is associated to a directed cycle in G with \(n_1\) vertices (\(n_1\ge 2\)) and assume without loss of generality that the vertices in G are numbered so that the first \(n_1\) nodes and \(n_1\) edges correspond to this cycle (\(1\rightarrow 2 \rightarrow \cdots \rightarrow n_1\rightarrow 1)\). If there is a hinge, assume \(n_1\) is the hinge. Then the first \(n_1\) columns of \(I_G\), the incidence matrix of G correspond to this cycle, and the last \(r-n_1\) columns have zeros in the first \(n_1-1\) entries:

We can consider the invertible \(n\times n\) block matrix \(K_1\) as follows:

By multiplying \(K_1\) and \(I_G\) we obtain the block matrix

whose last block equals the incidence matrix of the subgraph of G obtained by removing the first \(n_1-1\) vertices and the edges from the corresponding cycle. By an inductive argument we see that we can obtain finitely many invertible matrices \(K_1,K_2\ldots \) such that the product gives an invertible matrix \(K\in {\mathbb {Q}}^{n\times n}\) that yields the desired result. \(\square \)
We can now prove Theorems 6.6 and 6.7.
Proof of Theorem 6.6
We start by proving that G is lebn. As we pointed out in Remark 6.4, as there are no intermediate species, the equations of the mass-action system determined by G can be reconstructed from \(G_2^0\) in a mass-action fashion by treating the labels of the edges as if they were reaction constants. As the associated multidigraph \(MG_2\) does not have parallel edges between different nodes, these labels are monomials in the species concentrations.
Consider \(1\le \alpha \le d\). By the assumption of minimality of the partition, there is a connected component of \(G_2^0\), which we denote by \(H_\alpha \), with vertices the species in \({\mathscr {S}}^{(\alpha )}\). Consider, without loss of generality, that \({\mathscr {S}}^{(\alpha )}\) consists of the first \(n_\alpha \) species, i.e., \({\mathscr {S}}^{(\alpha )}=\{X_1,\ldots ,X_{n_\alpha }\}\). By Eq. (3.1) and Remark 6.4, we have
where we order the \(r_\alpha \) edges in \(H_\alpha \), \(R_{\kappa ,\alpha }(x)\) is the vector of size \(r_\alpha \) where if the i-th edge is \(X_k \overset{\kappa _i x^{\gamma _i}}{\longrightarrow } X_\ell \) then \(R_{\kappa ,\alpha }(x)_i = \kappa _i x_kx^{\gamma _i}\) and \(N_\alpha \) is the stoichiometric matrix which in this linear case coincides with the incidence matrix of \(H_\alpha \). Then, by multiplying \((f_1,\ldots ,f_{n_\alpha })^T \) on the left by the invertible matrix \(K_\alpha \) from Lemma 6.9, we obtain binomials in the monomials of \(R_{\kappa ,\alpha }(x)\).
By repeating the reasoning above with each \(1\le \alpha \le d\), we obtain a block matrix \(M\in {\mathbb {Q}}^{s\times s}\) that yields the binomial equivalence for \((f_1,\ldots ,f_s)\). To end the proof, it is immediate to see from Proposition 3.13 that any non-confluent extension \(G_C\) of G is also lebn. \(\square \)
Proof of Theorem 6.7
Assume that \(G_E\) has no directed cycles. We define the following subsets of indices, as in the proof of Theorem 3.15 in Pérez Millán and Dickenstein (2018):
It is important to note that \(L_0\ne \emptyset \) because the graph \(G_E\) has no directed cycles. For each \(\alpha \ge 1\), fix \(X_{i_\alpha }\in {\mathscr {S}}^{(\alpha )}\). Because of the minimality of the partition, any other \(X_i \in {\mathscr {S}}^{(\alpha )}\) lies in the connected component \(H_\alpha \) of \(G_2^0\) containing \(X_{i_\alpha }\).
Choose \(X_{i_{1}},\ldots ,X_{i_{d}}\) species, with \(X_{i_{\alpha }}\in {\mathscr {S}}^{(\alpha )}\), for each \(\alpha =1,\ldots ,d\). Take any other species \(X_{i}\in {\mathscr {S}}^{(\alpha )}\) for some \(\alpha \in L_k\), \(X_{i}\ne X_{i_{\alpha }}\), with \(L_k\) as above. As \(H_\alpha \) is strongly connected, let \(\rho (G)\) be the generator of the kernel of \({\mathcal {L}}(H_\alpha )\) as in (2.2). Then we can write \(x_i=\frac{\rho _i(H_\alpha )}{\rho _{i_\alpha }(H_\alpha )}x_{i_\alpha }\). But as \(H_\alpha \) is a directed cactus graph, there is a unique j-tree for each node j in \(H_\alpha \) and then each entry \(\rho _j(H_\alpha )\) is a monomial in the variables \(x_{i_{\beta }}\) with \({\beta }\in L_t\), with \(t<k\). Hence, the concentration of \(X_{i}\) can be expressed in terms of \(x_{i_1},\ldots ,x_{i_d}\) in the form:
for some \(\phi (\tau )\in {\mathbb {Q}}(\tau )\), where \(x^{a}\) is a Laurent monomial that depends only on variables \(x_{i_{\beta }}\) with \({\beta }\in L_t\), with \(t<k\). Note that, if \(k=0\), then \(x^{a}=1\).
Suppose that the species of G are ordered in the following way: first we put the species in \({\mathscr {S}}^{(\beta )}\), for all \(\beta \) such that \(\beta \in L_0\), then the species in \({\mathscr {S}}^{(\beta )}\), such that \(\beta \in L_1\), and so on. With this order, from Eq. (A.7) we obtain Laurent monomials of the form \(x_{i_{\alpha }}\, x^{a}\, x_i^{-1}\) with \(i\ne i_\alpha \), \(\alpha \in L_k\) and \(x^a\) involves variables \(x_j\) with \(j<i\) for any i such that \(X_i\in {\mathscr {S}}^{(\alpha )}\). From these monomials we build the \(d\times s\) matrix A as in Theorem 5.3.
On the other hand, there are d independent conservation relations by the hypotheses of the statement and Theorem 3.2 in Pérez Millán and Dickenstein (2018). They are:
Furthermore, \(\dim ({\mathcal {S}}^{\perp })=d\), where \({\mathcal {S}}\) is the stoichiometric subspace.
Consider the conservation-law matrix W according to the conservation laws (A.8). Note that by hypotheses and Proposition 5.6 in Pérez Millán and Dickenstein (2018), \(\textrm{rank}(W) + \textrm{rank}(\textrm{Exp})=s\). We will now apply Theorem 5.3. We note first that if we consider a submatrix of W with two columns corresponding to species in the same set \({\mathscr {S}}^{(\beta )}\), then, its determinant is zero, because the two columns are equal. So we are interested in submatrices with columns corresponding to species in different sets \({\mathscr {S}}^{(\beta )}\). Then, suppose that we choose the set \(I=\{j_1,\ldots ,j_d\}\) such that the species \(X_{j_{\beta }}\in {\mathscr {S}}^{(\beta )}\), for each \(\beta =1,\ldots ,d\). We then have \(W_I=Id_d\) and \(A_I\) is an upper triangular matrix with ones on its diagonal entries. Then, \(\det (W_I)\det (A_{I})=1\) in all these cases, and zero in the other cases, as we wanted to prove. \(\square \)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dickenstein, A., Giaroli, M., Pérez Millán, M. et al. Multistationarity questions in reduced versus extended biochemical networks. J. Math. Biol. 89, 18 (2024). https://doi.org/10.1007/s00285-024-02115-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-024-02115-7