Abstract
Despite the discovery of several bacteria capable of interacting with polymers, the activity of the natural bacterial isolates is limited. Furthermore, there is a lack of knowledge regarding the development of bioprocesses for polyethylene (PE) degradation. Here, we report a bioprocess using pseudo-resting cells for efficient degradation of PE. The bacterial strain Acinetobacter nosocomialis was isolated from PE-containing landfills and characterized using low-density PE (LDPE) surface oxidation when incubated with LDPE. We optimized culture conditions to generate catalytic pseudo-resting cells of A. nosocomialis that are capable of degrading LDPE films in a bioreactor. After 28 days of bioreactor operation using pseudo-resting cells of A. nosocomialis, we observed the formation of holes on the PE film (39 holes per 217 cm2, a maximum diameter of 1440 μm). This study highlights the potential of bacteria as biocatalysts for the development of PE degradation processes.
Key points
• New bioprocess has been proposed to degrade polyethylene (PE).
• Process with pseudo-resting cells results in the formation of holes in PE film.
• We demonstrated PE degradation using A. nosocomialis as a biocatalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyethylene (PE) is one of the most widely used synthetic plastics worldwide owing to its low cost, lightweight, and flexibility. However, the superior durability of PE causes long-term environmental damage due to its waste after use (Álvarez-Hernández et al. 2019; Foley et al. 2018; Gibb 2019; Rochman et al. 2016; Wei and Zimmermann 2017). PE is difficult to biodegrade naturally because of its highly stable C-H and C-C covalent bonds, hydrophobicity, and high molecular weight (Harshvardhan and Jha 2013). The current technology for PE waste treatment includes incineration, landfill, and chemical degradation, which have detrimental effects on the environment and humans (Jambeck et al. 2015; Yang et al. 2011).
Consequently, efforts to degrade PE waste have gained momentum (Raddadi and Fava 2019; Sanluis-Verdes et al. 2022; Yao et al. 2022a; Yoon and Jang 2021). PE biodegradation is a process performed by living organisms, including bacteria, fungi, algae, and invertebrates (Ghatge et al. 2020). However, the decomposition rate of PE using microorganisms is slow, with film surface oxidation requiring several months or longer (Otake et al. 1995). Previous studies have mostly reported partial oxidation of the PE film surface rather than complete decomposition (Restrepo-Flórez et al. 2014; Zahra et al. 2010). Additionally, the use of invertebrates in this process has yet to be explored, further limiting the options for rapid PE degradation (El-Sherif et al. 2022; Ghatge et al. 2020).
The slow rate of PE decomposition by microorganisms remains a challenge in advancing the study of biological degradation. Previous studies have attempted to address the slow rate of PE decomposition by applying pre-oxidation treatments, such as heat treatment, UV irradiation, and oxidation using nitric acid to enhance the biodegradation process. Although pre-oxidation treatments can improve the PE decomposition rate, they still require harsh conditions and separate non-biological processes (Brown et al. 1974; Cornell et al. 1984; Montazer et al. 2018; Volke-Sepúlveda et al. 2002; Yang et al. 2014).
Bacteria have a well-established history in bioprocess development (Dodds and Gross 2007; Liew et al. 2022). Some engineered bacteria, including the genera Escherichia, Corynebacterium, and Clostridium, have a long record of use in bioprocesses to produce compounds such as amino acids and solvents (Carneiro et al. 2013; Jang et al. 2012a, 2012b; Kitade et al. 2018; Woo et al. 2019). In contrast, some PE-degrading bacteria, including Bacillus, Arthrobacter, Flavobacterium, Nocardia, Ralstonia, and Pseudomonas, have been shown to utilize PE as a carbon source for growth (Koutny et al. 2009, 2006b; Nowak et al. 2011; Tribedi and Sil 2013). To the best of our knowledge, no previous research has reported the development of bioprocesses using these bacteria to enhance PE degradation in bioreactors. In this study, we develop a bioreactor-based process for enhancing the degradation rate of PE using bacteria.
Materials and methods
Microbial strain
The microbial strain used in this study was Acinetobacter nosocomialis GNU001, which was isolated from plastic-containing landfills. The strain was deposited under the Korean Collection for Type Cultures (KCTC) accession number KCTC 18879P. For the detailed isolation process, refer to the sections below.
Collection of PE waste for bacterial isolation
PE waste was obtained from plastic-containing landfills. Film waste was collected from a landfill containing 30-year-old PE films used in greenhouses during relocation and rearrangement processes. The waste had been buried for over 20 years and was recently excavated and exposed to the ground for more than 2 years. Sterilized 50-mL conical tubes were used to gather waste PE samples that were exposed on the ground surface. The collected samples were immediately used for microbial isolation, while the remaining pieces were cut into small fragments, stored at − 80 °C, and later used for additional bacterial isolation in this study.
Isolation of PE degradation bacteria
The collected PE waste sample was placed in a test tube containing 10 mL modified Luria Bertani (LB) broth. The modified LB contains 1.25 g/L of yeast extract, 10 g/L of peptone, and 10 g/L of sodium chloride. The sample was incubated at 37 °C overnight with shaking at 180 rpm in an incubator (IST-4075, JeioTech, Korea). After removing the soil particles through a brief centrifugation, the broth was diluted 1000 times and spread onto carbon-free minimal (CFM) agar plates supplemented with 10 g/L LDPE powder (S/N 5204091500405, M.J POWDER, Korea). This quantity of LDPE powder was determined to evenly cover the entire plate, ensuring that the powder could come into contact with the bacterial colonies on the surface. The CFM medium was prepared by adding 0.2% ammonium sulfate, 1% trace elements (0.1% FeSO4·7H2O, 0.1% MgSO4·7H2O, 0.01% CuSO4·5H2O, 0.01% MnSO4·5H2O, and 0.01% ZnSO4·7H2O), and 10 mM potassium phosphate (pH 7.0) per liter. When preparing agar plates, we used 15 g/L agar powder. The CFM medium was prepared by slight modification of the medium used for the isolation of poly(ethylene terephthalate) degrading microorganisms (Seong et al. 2023; Yoshida et al. 2016). The plates were incubated at 37 °C using an incubator (1B-15G, JeioTech, Korea) for a period of 12–48 h. The resulting colonies were subcultured three times on fresh CFM agar plates supplemented with LDPE powder to ensure pure cultures. The selected colonies were inoculated into CFM broth supplemented with LDPE film (Cat. No. ET311130, Goodfellow) and incubated for 7 months to confirm the final strain. The final strain cultured for 7 months was made into a stock and stored at − 80 °C, which was used for subsequent experiments.
Identification of PE-degrading bacteria isolate
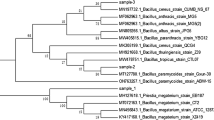
The stocked bacterial cells were cultured in modified LB broth for total DNA isolation. After harvesting the cells by centrifugation at 13,000 rpm (IST-4075; JeioTech, Korea), total DNA was extracted using a G-spin™ Genomic DNA Extraction Kit (iNtRON Biotechnology, Korea). The 16S rRNA gene was amplified from the total DNA as a template by using primer pairs 27-F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492-R (5′- GGTTACCTTGTTACGACTTC-3′) (Kim et al. 2022; Ranjbar et al. 2022). The bacterial isolates were identified by sequencing the PCR products (Cosmo Genetech, Korea). Homology comparisons of 16S rRNA sequences were performed using NCBI BLASTN (Kang et al. 2022). A neighbor-joining phylogenetic tree was constructed using MEGA-X software (Lee et al. 2021; Park et al. 2021).
Optimization of culture conditions for A. nosocomialis isolate
The growth of A. nosocomialis GNU001 cells in LB broth was examined under varying temperatures (25, 30, and 37 °C) and pH (5, 6, 7, 8, and 9) conditions. The maximum cell density of A. nosocomialis was measured in cultures containing chemically defined CFM medium supplemented with varying carbon and nitrogen sources. In the carbon source tests, the CFM medium was supplemented with various carbon sources, including glucose, maltose, glycerol, saccharose, fructose, lactose, and starch, each at a concentration of 10 g/L. Similarly, for the nitrogen source tests, the CFM medium contained various nitrogen sources, such as yeast extract, peptone, tryptone, ammonium chloride, ammonium sulfate, and ammonium nitrate, each at a concentration of 10 g/L. Maximum cell density and specific growth rate (μmax) were calculated from the growth profiles. Cell density was measured at 600 nm using a Bioscreen C instrument (Oy Growth Curves Ab Ltd., Helsinki, Finland) or a microplate reader (Infinite 200 Pro, Tecan, Switzerland), following the manufacturer’s protocol.
Fourier transform infrared (FT-IR) spectroscopy
The functional groups present on the PE film surface were determined using attenuated total reflectance FT-IR transform infrared (ATR-FTIR) spectroscopy using an FT-IR spectrometer (IS50, Thermo Fisher, Waltham, USA). The PE film specimens were first cleaned by soaking in 2% SDS for 1 day, followed by washing twice with sterile water and finally once with ethanol. The dried PE film specimens were analyzed using an FT-IR spectrometer with a range of 4000–400 cm−1.
X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectra were collected using an XPS spectrometer (K-ALPHA, Thermo Fisher Scientific, Waltham, MA, USA) to determine the presence of carbon–carbon, carbon–oxygen, and carbon-hydrogen bonds. The C1s peak was analyzed by fitting it to the range of 280–300 eV. The sample preparation process for XPS analysis was the same as that for the FT-IR analysis, involving soaking in 2% SDS for 1 day, washing twice with sterile water and once with ethanol, and drying the PE film specimen.
Contact angle
The hydrophobicity of the PE films was evaluated by measuring the water contact angle using a Phoenix 300 Touch instrument (SEO, Korea) (Novotný et al. 2018). The water contact angle was measured at five different regions of each PE film. The preparation process for the PE film specimens for the contact angle analysis was the same as that used for the FT-IR and XPS analyses.
Light transmittance
Transmittance spectra of the PE film specimens were recorded using a UV/Vis spectrophotometer (V-730, JASCO, Japan) at the KOPTRI Institute (Seoul, South Korea). The spectra were obtained in the 200–1100 nm at a scan rate of 400 nm/min (Singh et al. 2021). The specimens for this analysis were prepared using the same method as that used for surface functional group analysis.
Surface morphology
The surface morphology of the PE film samples was analyzed using a polarizing microscope (BX50, Olympus, Tokyo, Japan) to assess physical changes such as the formation of holes, cracks, and roughness on the surface. The presence of biofilms on the PE film surface was analyzed using a scanning electron microscopy (SEM; JSM-7610F, JEOL, Japan) after the samples were coated with gold.
Bioreactor conditions
A bioreactor consisting of a 5-L jar (MARADO-05S-PB, CNS, Daejeon, Korea) was used to determine the optimal conditions for preparing pseudo-resting cells and to demonstrate the improved degradation of the PE film. The bioreactor was sterilized at 121 °C for 15 min and was equipped with calibrated probes for sensing dissolved oxygen (DO) and pH. Unless specified otherwise, the fermentation was carried out in a 2.5-L working volume using either LB or CFM broth. When using LB medium, DO control in the bioreactor was achieved through the DO stat mode, which automatically regulates agitation. At that time, the agitation speed was controlled within a range of 100–500 rpm, ensuring a DO level of 50% or higher at an airflow rate of 5 L/min. In contrast, when the CFM medium was used, aeration was controlled to maintain a DO level of at least 50% by adjusting only the airflow ranging from 2 to 5 L/min. In addition, PE films (3.5 cm × 20 cm and 3.5 cm × 7 cm) were added to the CFM medium as a carbon source.
Preparation of pseudo-resting cells
A single colony of A. nosocomialis was grown first in 5 mL of LB broth in a test tube overnight. Subsequently, 1 mL of this overnight culture was transferred to a 1-L flask containing 250 mL of LB medium, and the cells were further cultured at 37 °C and 200 rpm until the OD600 reached approximately 1 (1.38 × 108 cells/mL). This culture was used as an inoculum for a bioreactor containing 3.75 L of LB medium. After reaching a cell density of approximately 1 (OD600), the harvesting process involved centrifugation of the broth at 6000 rpm for 10 min at 4 °C after being chilled on ice, followed by washing twice with CFM medium. In this study, for LDPE film decomposition employing pseudo-resting cells, we used cells obtained from a 3.4-L culture with an OD600 of 1, which we refer to as 3400 optical density units (ODU). The harvested cells equivalent to 3400 ODU were introduced into the main bioreactor (resulting in an OD600 of approximately 1.36). This bioreactor contained 2.5 L of CFM medium supplemented with LDPE films, and the viable cells were employed as pseudo-resting cells.
Results
Isolation and identification of LDPE-degrading bacterial strains
To isolate a PE-degrading aerobic bacterium, we collected surface-exposed PE fragments from landfills. During the first stage of screening using PE fragment extracts, 37 colonies of varying morphologies were observed on agar plates containing CFM media with LDPE powder as the sole carbon source. The isolates were screened further using repeated sub-culturing methods. In the final stage, three different colonies were obtained and inoculated into CFM broth supplemented with the LDPE film. A control test tube containing the same medium and LDPE fragments was prepared without inoculating any bacterial cells. After 7 months of culture, we obtained a single bacterial isolate, named GNU001, which was capable of growing in broth containing the LDPE film (Fig. S1a). Cell lysis was not observed throughout the culture periods. No bacterial growth was observed in the test tubes inoculated with the other two colonies or in the control test tubes (Fig. S1b). Further studies were conducted using a film incubated with bacterial isolate GNU001, which was identified as A. nosocomialis by 16S rRNA sequencing, and the bacterium itself (Fig. 1a).
a Phylogenetic tree showing the identification of the bacterial isolate GNU001 as A. nosocomialis through 16S rRNA sequencing. b SEM image of the LDPE film surface after 7 months of incubation with A. nosocomialis GNU001 showing biofilm formation. c SEM image (control). d ATR-FTIR spectra showing the functional groups on the surface of PE film obtained from the control culture (black) and the culture with A. nosocomialis GNU001 (red). e XPS spectra indicating a significant difference in the peak at 286.9 eV (indicating C-O groups) in the comparison between the C1s spectra of the PE specimens obtained from the control and microbial cultures with GNU001 strain, supporting the oxidation of the PE film surface
First, to examine the formation of biofilms, which is known to be an initial step in activating the biodegradation process (Donlan 2002; Joshi et al. 2022; Koutny et al. 2006a; Roy et al. 2008), we observed the surface of an LDPE film incubated for 7 months with the A. nosocomialis GNU001 isolate using SEM (Fig. 1b). SEM images showed that the surfaces of the LDPE film samples incubated with the bacterial isolate formed a biofilm containing A. nosocomialis cells (Fig. 1b), unlike the LDPE film from the control culture, which showed a different morphology (Fig. 1c).
Next, the biofilm and cells were thoroughly removed using 2% SDS to assess changes in the functional groups on the surface of the LDPE film where the biofilm was formed. The functional groups on the surface of the PE film were analyzed using ATR-FTIR and XPS (Fig. 1f, g). ATR-FTIR analysis revealed four peaks at 2914, 2848, 1462, and 719 cm−1 in the spectrum of the LDPE film obtained from the cell-free control culture. These four peaks correspond to the intrinsic bonds present in the PE film, such as C-H bond stretching, C-H bond bending, and CH2 bond rocking, respectively (Chércoles Asensio et al. 2009; Krimm et al. 1956). In contrast, on the surface of the LDPE film incubated with A. nosocomialis cells, four new peaks were identified at 3304, 1034, and 1655 cm−1 in addition to the four peaks observed in the control film (Fig. 1f). The broad peak at 3304 cm−1, ranging from 2981 to 3775, is an indication of the hydroxyl group, while the peak at 1034 cm−1 represents C-O stretching (Sridharan et al. 2021). The peak at 1655 cm−1 is widely recognized to be associated with the carbonyl group (Dayma and Satapathy 2012). The findings indicate that the presence of A. nosocomialis cells resulted in the oxidation of the carbon backbone of the LDPE film surface, leading to the formation of oxygen-containing functional groups.
This was also confirmed using XPS analysis, which is commonly used to evaluate the surface chemical composition and elemental content (Fig. 1g). The significant difference in the peak at 286.9 eV in the comparison between the C1s spectra of the LDPE specimens obtained from the control and microbial cultures with the GNU001 strain supports the oxidation of the LDPE film surface (Fig. 1g). The presence of C-O groups at 286.9 eV in the XPS analysis is commonly associated with the presence of alcohols, ethers, and carboxylic acids (Zhu et al. 2001). In summary, the results suggest that A. nosocomialis GNU001 adhered to the surface of the LDPE film by forming a biofilm, resulting in the oxidation of the surface.
Optimization of culture conditions for the pseudo-resting cell development
Although the A. nosocomialis GNU001 strain has the potential to oxidize the LDPE film surface, it requires 7 months, which means that it takes a significant amount of time for the LDPE film to be efficiently degraded by microbial activity alone. We presume that the slow degradation of the LDPE film by the A. nosocomialis isolation was due to either one or a combination of two factors. The first factor is the low growth rate of A. nosocomialis cells when the LDPE film is the sole carbon source. The second factor is the sluggish release of small molecules from LDPE film that the isolated GNU001 could use as an energy source. Indeed, we observed a low cell density (OD600) of less than 0.01 after 7 months of culture of the GNU001 strain in a test tube containing 10 mL of CFM medium, a chemically defined medium supplemented with an LDPE film fragment by single colony inoculation (Fig. S1a).
To facilitate efficient PE decomposition, it was crucial to establish an appropriate initial count of A. nosocomialis cells as a biocatalyst. In this context, we employed the concept of “pseudo-resting cells” to characterize the state of microbial cells when employed as whole-cell catalysts for LDPE decomposition. Conventionally, resting cells refer to microbial cells in a non-growing state, commonly utilized as biocatalysts in whole-cell biotransformation processes. In this study, LDPE, as a sole carbon source, being a polymer, limits the release of available carbon sources in the given environment, leading to the “pseudo-resting” state of A. nosocomialis cells.
To optimize the conditions for the development of the pseudo-resting cells, the growth properties of A. nosocomialis GNU001 were first studied under various temperatures and pH conditions in rich LB media (Fig. 2a and b). The maximum cell densities of A. nosocomialis GNU001 were similar under temperatures of 25, 30, and 37 ℃ when grown in LB broth (Fig. 2a, gray bars). However, the highest maximum specific growth rate (μmax) of 1.55 was observed at 37 ℃ (Fig. 2a, white bars). A. nosocomialis cells showed consistent growth up to an OD600 of 1.65 at a pH range of 5 to 9, in LB broth at 25 °C (Fig. 2b). However, cell growth in chemically defined CFM medium supplemented with varying carbon and nitrogen sources was less than that obtained from the cultures in LB media (Fig. 2c and d). These results suggest that A. nosocomialis isolates grow well in LB broth at a pH range of 5 to 9 and mesophilic temperatures, indicating the proper conditions for develo** the pseudo-resting cells.
a–d Optimization of culture conditions for the development of pseudo-resting cells of A. nosocomialis GNU001. e Schematic representation of the development of pseudo-resting cells of A. nosocomialis GNU001 by transferring the precultured cells in LB broth into a 2.5-L main bioreactor containing chemically defined CFM medium supplemented with LDPE films. f The optimal cell quantities for develo** pseudo-resting cells were assessed by transferring harvested A. nosocomialis cells with ODU values of 850, 1700, and 3400 into the main bioreactor containing 2.5 L CFM medium supplemented with LDPE films. The ODU values of 850, 1700, and 3400 correspond to the cell equivalents obtained from cultures of 0.85 L, 1.7 L, and 3.4 L, each with an OD600 of 1. Symbols indicate ODU values of 850 (triangle), 1700 (open square), and 3400 (black square)
It was considered appropriate to use pseudo-resting cells in chemically defined media rather than resting cells in non-growth media because the degradation of LDPE takes several days to weeks. To ascertain the optimal cell quantities for pseudo-resting cell development, harvested A. nosocomialis cells equivalent to ODU values of 850, 1700, and 3400 were transferred into a main bioreactor containing 2.5 L CFM medium supplemented with LDPE films. Following cell transfer, the initial OD600 values were measured at 0.34, 0.68, and 1.36, respectively (Fig. 2e and f). Subsequently, all bioreactors containing cells at 850, 1700, and 3400 ODU encountered a decline in cell density attributed to lysis (Fig. 2f). Notably, there was no significant cell recovery observed in the bioreactors with 850 and 1700 ODU cells. However, in the bioreactors with 3400 ODU-transferred cells, a recovery of up to 0.492 (OD600) was observed at 68 h (Fig. 2f). This suggests that among the tested ODU values, only the cells recovered in the bioreactor containing 3400 ODU-transferred cells are suitable as pseudo-resting cells for the LDPE decomposition process.
Enhancement of LDPE film decomposition in the bioreactor using the pseudo-resting cells of A. nosocomialis
LDPE film decomposition was performed in a 2.5-L bioreactor with chemically defined CFM media and was catalyzed by the pseudo-resting cells of A. nosocomialis (Fig. 3a and b). Cell density then increased to 0.66 (OD600) at 211 h and was sustained until 521 h (Fig. 3c). The catalytic reactions were continued using the pseudo-resting cells of A. nosocomialis until they entered the death phase, which was monitored for 672 h (28 days). As a control, another bioreactor was operated using Escherichia coli cells under the same conditions.
a, b The 2.5-L bioreactor used for LDPE film decomposition. c Cell density profiles of A. nosocomialis GNU001 pseudo-resting cells during 28 days of bioreactor operation in chemically defined CFM media supplemented with LDPE film. d, e Morphological analysis of LDPE film specimens after 28 days of bioreactor operation: LDPE film specimens incubated with either E. coli (control; d) or A. nosocomialis GNU001 (e) (see Fig S6 for the high-resolution images). The morphology of the specimens was observed at 200 × magnification using a polarizing microscope
After 28 days of operation in the bioreactor, the LDPE film specimens were analyzed by studying light transmittance, contact angle, XPS, and microscopy data. A slight increase in the opacity of the LDPE film specimens incubated with A. nosocomialis GNU001 cells was observed, which became more pronounced when the films were layered in triplicate (Fig. 4). Light transmittance measurements confirmed an increase in the opacity of the LDPE film specimens (Fig. 5). The transmittance of the LDPE film specimens incubated with A. nosocomialis decreased in all ultraviolet (UV; 200–380 nm), visible (380–780 nm), and near-infrared (NIR; > 780 nm) spectra tested, compared to that of the control film (Fig. 5). The decrease in the transmittance was more pronounced in the UV region than in the NIR region (Fig. 5).
The surface of the LDPE film specimen was characterized by contact angle and XPS analyses because of the suspected increase in opacity resulting from changes on the film surface. The water contact angle of the LDPE film from the bioreactor containing A. nosocomialis cells was 93.7°, which was a decrease of 6% compared to that of the control film (Fig. 6). This suggests that the surface of the LDPE film specimens from the bioreactor containing A. nosocomialis cells was more hydrophilic than that of the control film. XPS analysis revealed that the LDPE film specimens from the bioreactor with A. nosocomialis cells had an elevated oxygen content compared to the control film. This was demonstrated by an O1s/C1s ratio of 20%, which was 4.3 times greater than that of the control film (Fig. 7). These findings were indicated by the C-O and C = O peaks at 286.4 eV and 288.7 eV, respectively. Notably, the peak at 288.7 eV, representing C = O, was only detected on the surface of the PE film specimens obtained from the bioreactor containing A. nosocomialis cells (Fig. 7).
C1s XPS spectra of the PE specimens obtained from cultures with A. nosocomialis supporting the surface functional groups, including C-O (286.4 eV) and C = O (288.7 eV). The C = O group was only detected on the surface of the LDPE film specimens obtained from the bioreactor containing A. nosocomialis cells
Finally, the morphology of the LDPE film specimens was analyzed at 200 × magnification using polarizing microscopy (Fig. 3d and e; see Fig. S2 for the high-resolution images). The control film specimens appeared smooth with a few scratches, possibly due to the casting process (Fig. 3d; also see Fig. S2 for the high-resolution images). Meanwhile, rough surface structures were observed on the LDPE film specimens obtained from the bioreactor containing A. nosocomialis cells under a microscope at 200 × magnification (Fig. 3e; also see Fig. S2 for the high-resolution images). Furthermore, the LDPE specimens obtained from the bioreactor containing A. nosocomialis cells contained holes that were not present in the control LDPE film (Fig. 3d, e; also see Fig. S2 for the high-resolution images). The analysis of the holes in a 217 cm2 LDPE film specimen showed that there were approximately 39 holes in total, with the largest measuring 1440 μm in diameter. The distribution of hole diameters was even, ranging from 13.5 to 1440 μm (Fig. 8). The experiments with 7-month cultures after A. nosocomialis colony inoculation resulted in partial oxidation of the LDPE surface, whereas the results obtained from pseudo-resting cells showed a significant acceleration of LDPE degradation. Furthermore, as holes have not been reported in LDPE degradation using bacteria, the findings of this study hold promise for advancing our understanding of process development for polymer waste decomposition in future studies.
Discussion
The environmental impact of PE waste is an important concern because PE is one of the most widely used and abundant plastics in the world. PE waste is not easily biodegradable and has persisted in the environment for hundreds of years, contributing to environmental pollution. Finding sustainable solutions for PE waste management is crucial for reducing its environmental impact (Aryan et al. 2019). In this study, we isolated LDPE-degrading bacteria under aerobic conditions, and designed and implemented strategies to enhance LDPE degradation using pseudo-resting cells.
The biodegradation of LDPE by bacteria has been a subject of interest in recent decades, and many bacterial strains have been found to interact with the polymer (Biki et al. 2021; El-Sherif et al. 2022; Joshi et al. 2022). Several genera, including Bacillus, Lysinibacillus, and Marinobacter, have been shown to have the potential to degrade LDPE and are widely distributed across various environments, such as landfills, soil, the marine environment, and the gut of insects (Jeon et al. 2021; Khandare et al. 2022; Vimala and Mathew 2016). For instance, some LDPE-degrading bacteria have been isolated from landfills, including A. nosocomialis isolated under aerobic conditions in this study, and Paneibacilus sp. and Aspergillus clavatus strains reported in previous studies Lysinibacillus sp. and Pseudomonas aeruginosa were obtained from soil, whereas Idiomarina sp., Marinobacter sp., and Exiguobacterium sp. were obtained from marine environments (Gao and Sun 2021; Jeon et al. 2021; Yao et al. 2022b). Some species of Acinetobacter and Bacillus have been found in the gut of Tenebrio molitor larvae (Yin et al. 2020). This emphasizes the widespread presence of LDPE-degrading bacteria in different environments, making them readily accessible.
However, the degradation activity of these naturally isolated bacteria is limited (El-Sherif et al. 2022; Otake et al. 1995). For example, Lysinibacillus has been reported to increase the roughness of PE surfaces, detect oxidized functional groups, and cause a 9% decrease in LDPE weight (Yao et al. 2022b). Similarly, Acinetobacter and Bacillus have been reported to cause an 18% reduction in LDPE weight after 30 days of cultivation (Yin et al. 2020). The A. nosocomialis isolate in this study also demonstrated oxidation of the LDPE film surface during simple culture (Fig. 3a–c). While the oxidation and weight reduction of LDPE specimens have been reported, no direct evidence, such as visible holes, has been reported in studies using bacteria. Therefore, it is worth noting that hole formation in PE by bacteria represents a novel finding reported for the first time in this study. In the case of eukaryotes, recent studies have reported the pore formation of PE by fungi and the digestion of PE by Galleria mellonella (Bombelli et al. 2017; Gao et al. 2022).
To overcome the limitations of bacteria as catalysts for LDPE decomposition, various pretreatments such as heat treatment and UV irradiation have been investigated (Jeon and Kim 2014). In addition, develo** a bioprocess that can efficiently utilize LDPE as the carbon source without physical pretreatment is crucial. In this study, the concept of resting cells, which is commonly utilized in biotransformation to produce target compounds from bacterial precursors (De Carvalho et al. 2005; Kao et al. 2003; Narancic et al. 2013), was applied to LDPE degradation for the first time. The idea of using bacteria as catalysts remains the same; however, a difference between converting a precursor into a target compound and decomposing the LDPE has been followed. In this study, we successfully demonstrated enhanced LDPE decomposition using a pseudo-resting cell, similar to the resting cell process, after optimizing these processes. This led to the formation of holes in the LDPE film (Fig. 3d and e). While this study serves as a proof-of-concept, further studies are required to optimize the conditions for process development using resting or pseudo-resting cells. Future studies to enhance the PE-degradation capabilities of bacteria that serve as catalysts may involve systems metabolic engineering and synthetic biology. Moreover, incorporating various pretreatment methods into the process using the pseudo-resting cells for PE degradation is expected to yield a synergistic effect in terms of degradation.
In this study, we demonstrate a novel bioprocess for LDPE degradation using pseudo-resting cells of the bacterial strain A. nosocomialis isolated from a landfill. The results of ATR-FTIR and XPS analyses showed that the incubation of LDPE with A. nosocomialis GNU001 led to an increase in the oxygen-containing functional groups and hydrophilicity of the LDPE films, which is evidence of the surface being oxidized. Bioreactor operation with pseudo-resting cells of A. nosocomialis GNU001 was accelerating degradation of LDPE, as indicated by the observation of holes in the LDPE film and its surface roughness under microscopic examination. This proof-of-concept study offers a promising approach to enhance PE degradation and contributes to solving the global plastic waste problem. Further optimization and improvement of bioprocesses using the resting cell concept or microbial strain improvement through systems metabolic engineering and synthetic biology are necessary to achieve more efficient PE degradation.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Álvarez-Hernández C, Cairós C, López-Darias J, Mazzetti E, Hernández-Sánchez C, González-Sálamo J, Hernández-Borges J (2019) Microplastic debris in beaches of Tenerife (Canary Islands, Spain). Mar Pollut Bull 146:26–32
Aryan Y, Yadav P, Samadder SR (2019) Life cycle assessment of the existing and proposed plastic waste management options in India: a case study. J Clean Prod 211:1268–1283. https://doi.org/10.1016/j.jclepro.2018.11.236
Biki SP, Mahmud S, Akhter S, Rahman MJ, Rix JJ, Al Bachchu MA, Ahmed M (2021) Polyethylene degradation by Ralstonia sp. strain SKM2 and Bacillus sp. strain SM1 isolated from land fill soil site. Environ Technol Innov 22:101495. https://doi.org/10.1016/j.eti.2021.101495
Bombelli P, Howe CJ, Bertocchini F (2017) Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr Biol 27(8):R292–R293. https://doi.org/10.1016/j.cub.2017.02.060
Brown BS, Mills J, Hulse JM (1974) Chemical and biological degradation of waste plastics. Nature 250(5462):161–163
Carneiro S, Ferreira EC, Rocha I (2013) Metabolic responses to recombinant bioprocesses in Escherichia coli. J Biotechnol 164(3):396–408. https://doi.org/10.1016/j.jbiotec.2012.08.026
Chércoles Asensio R, San Andrés Moya M, de la Roja JM, Gómez M (2009) Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal Bioanal 395(7):2081–2096. https://doi.org/10.1007/s00216-009-3201-2
Cornell JH, Kaplan AM, Rogers MR (1984) Biodegradability of photooxidized polyalkylenes. J Appl Polym Sci 29(8):2581–2597
Dayma N, Satapathy BK (2012) Microstructural correlations to micromechanical properties of polyamide-6/low density polyethylene-grafted-maleic anhydride/nanoclay ternary nanocomposites. Mater Des 33:510–522. https://doi.org/10.1016/j.matdes.2011.04.057
De Carvalho CCCR, Poretti A, da Fonseca MMR (2005) Cell adaptation to solvent, substrate and product: a successful strategy to overcome product inhibition in a bioconversion system. Appl Microbiol Biotechnol 69(3):268–275. https://doi.org/10.1007/s00253-005-1967-5
Dodds DR, Gross RA (2007) Chemicals from biomass. Science 318(5854):1250–1251. https://doi.org/10.1126/science.1146356
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881
El-Sherif DM, Eloffy MG, Elmesery A, Abouzid M, Gad M, El-Seedi HR, Brinkmann M, Wang K, Al Naggar Y (2022) Environmental risk, toxicity, and biodegradation of polyethylene: a review. Environ Sci Pollut Res 29:54. https://doi.org/10.1007/s11356-022-23382-1
Foley CJ, Feiner ZS, Malinich TD, Höök TO (2018) A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci Total Environ 631:550–559
Gao R, Sun C (2021) A marine bacterial community capable of degrading poly(ethylene terephthalate) and polyethylene. J Hazard Mater 416:125928. https://doi.org/10.1016/j.jhazmat.2021.125928
Gao R, Liu R, Sun C (2022) A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene. J Hazard Mater 431:128617. https://doi.org/10.1016/j.jhazmat.2022.128617
Ghatge S, Yang Y, Ahn J-H, Hur H-G (2020) Biodegradation of polyethylene: a brief review. Appl Biol Chem 63(1):27. https://doi.org/10.1186/s13765-020-00511-3
Gibb BC (2019) Plastics are forever. Nat Chem 11(5):394–395 https://doi.org/10.1038/s41557-019-0260-7
Harshvardhan K, Jha B (2013) Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea. India Mar Pollut Bull 77(1–2):100–106
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347(6223):768–771. https://doi.org/10.1126/science.1260352
Jang Y-S, Kim B, Shin JH, Choi YJ, Choi S, Song CW, Lee J, Park HG, Lee SY (2012a) Bio-based production of C2–C6 platform chemicals. Biotechnol Bioeng 109(10):2437–2459. https://doi.org/10.1002/bit.24599
Jang Y-S, Lee ** Y, Lee J, Park ** H, Im Jung A, Eom M-H, Lee J, Lee S-H, Song H, Cho J-H, Seung Do Y, Lee Sang Y (2012) Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum. mBio 3(5):00314–12. https://doi.org/10.1128/mBio.00314-12
Jeon HJ, Kim MN (2014) Degradation of linear low density polyethylene (LLDPE) exposed to UV-irradiation. Eur Polym J 52:146–153. https://doi.org/10.1016/j.eurpolymj.2014.01.007
Jeon J-M, Park S-J, Choi T-R, Park J-H, Yang Y-H, Yoon J-J (2021) Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polym Degrad Stab 191:109662. https://doi.org/10.1016/j.polymdegradstab.2021.109662
Joshi G, Goswami P, Verma P, Prakash G, Simon P, Vinithkumar NV, Dharani G (2022) Unraveling the plastic degradation potentials of the plastisphere-associated marine bacterial consortium as a key player for the low-density polyethylene degradation. J Hazard Mater 425:128005. https://doi.org/10.1016/j.jhazmat.2021.128005
Kang J-Y, Park W-J, Yoon Y, Kim B-G (2022) Production of isoquercitrin from quercetin by biotransformation using Bascillus sp. CSQ10 isolated from Camellia sinensis cultivation soils. Appl Biol Chem 65(1):59. https://doi.org/10.1186/s13765-022-00727-5
Kao CM, Liu JK, Lou HR, Lin CS, Chen SC (2003) Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 50(8):1055–1061. https://doi.org/10.1016/S0045-6535(02)00624-0
Khandare SD, Agrawal D, Mehru N, Chaudhary DR (2022) Marine bacterial based enzymatic degradation of low-density polyethylene (LDPE) plastic. J Environ Chem Eng 10(3):107437. https://doi.org/10.1016/j.jece.2022.107437
Kim J, Kim K-Y, Ko JK, Lee S-M, Gong G, Kim KH, Um Y (2022) Characterization of a novel acetogen Clostridium sp. JS66 for production of acids and alcohols: focusing on hexanoic acid production from syngas. Biotechnol Bioprocess Eng 27(1):89–98. https://doi.org/10.1007/s12257-021-0122-1
Kitade Y, Hashimoto R, Suda M, Hiraga K, Inui M (2018) Production of 4-hydroxybenzoic acid by an aerobic growth-arrested bioprocess using metabolically engineered Corynebacterium glutamicum. Appl Environ Microbiol 84(6):e02587-e2617. https://doi.org/10.1128/AEM.02587-17
Koutny M, Lemaire J, Delort A-M (2006a) Biodegradation of polyethylene films with prooxidant additives. Chemosphere 64(8):1243–1252. https://doi.org/10.1016/j.chemosphere.2005.12.060
Koutny M, Sancelme M, Dabin C, Pichon N, Delort A-M, Lemaire J (2006b) Acquired biodegradability of polyethylenes containing pro-oxidant additives. Polym Degrad Stab 91(7):1495–1503. https://doi.org/10.1016/j.polymdegradstab.2005.10.007
Koutny M, Amato P, Muchova M, Ruzicka J, Delort A-M (2009) Soil bacterial strains able to grow on the surface of oxidized polyethylene film containing prooxidant additives. Int Biodeterior Biodegr 63(3):354–357. https://doi.org/10.1016/j.ibiod.2008.11.003
Krimm S, Liang CY, Sutherland GBBM (1956) Infrared spectra of high polymers. II Polyethylene J Chem Phys 25(3):549–562. https://doi.org/10.1063/1.1742963
Lee JH, Lee HY, Cho DY, Kim MJ, Jung JG, Jeong EH, Haque MA, Cho KM (2021) Biodegradable properties of organophosphorus insecticides by the potential probiotic Lactobacillus plantarum WCP931 with a degrading gene (opdC). Appl Biol Chem 64(1):62. https://doi.org/10.1186/s13765-021-00632-3
Liew FE, Nogle R, Abdalla T, Rasor BJ, Canter C, Jensen RO, Wang L, Strutz J, Chirania P, De Tissera S, Mueller AP, Ruan Z, Gao A, Tran L, Engle NL, Bromley JC, Daniell J, Conrado R, Tschaplinski TJ, Giannone RJ, Hettich RL, Karim AS, Simpson SD, Brown SD, Leang C, Jewett MC, Köpke M (2022) Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat Biotechnol 40(3):335–344. https://doi.org/10.1038/s41587-021-01195-w
Montazer Z, Habibi-Najafi MB, Mohebbi M, Oromiehei A (2018) Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading bacteria isolated from plastic-dump soil. J Polym Environ 26(9):3613–3625
Narancic T, Radivojevic J, Jovanovic P, Francuski D, Bigovic M, Maslak V, Savic V, Vasiljevic B, O’Connor KE, Nikodinovic-Runic J (2013) Highly efficient Michael-type addition of acetaldehyde to β-nitrostyrenes by whole resting cells of Escherichia coli expressing 4-oxalocrotonate tautomerase. Bioresour Technol 142:462–468. https://doi.org/10.1016/j.biortech.2013.05.074
Novotný Č, Malachová K, Adamus G, Kwiecień M, Lotti N, Soccio M, Verney V, Fava F (2018) Deterioration of irradiation/high-temperature pretreated, linear low-density polyethylene (LLDPE) by Bacillus amyloliquefaciens. Int Biodeterior Biodegr 132:259–267. https://doi.org/10.1016/j.ibiod.2018.04.014
Nowak B, Pająk J, Drozd-Bratkowicz M, Rymarz G (2011) Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int Biodeterior Biodegr 65(6):757–767. https://doi.org/10.1016/j.ibiod.2011.04.007
Otake Y, Kobayashi T, Asabe H, Murakami N, Ono K (1995) Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J Appl Polym Sci 56(13):1789–1796. https://doi.org/10.1002/app.1995.070561309
Park BH, Kim IS, Park JK, Zhi Z, Lee HM, Kwon OW, Lee BC (2021) Probiotic effect of Lactococcus lactis subsp. cremoris RPG-HL-0136 on intestinal mucosal immunity in mice. Appl Biol Chem 64(1):93. https://doi.org/10.1186/s13765-021-00667-6
Raddadi N, Fava F (2019) Biodegradation of oil-based plastics in the environment: existing knowledge and needs of research and innovation. Sci Total Environ 679:148–158
Ranjbar HH, Abari AH, Ghasemi SM, Ghorbani N (2022) Antioxidant and anticancer effects of epsilon-poly-L-lysine produced by two novel strains of Paenibacillus polymyxa HS6 and Stenotrophomonas maltophilia YS8. Biotechnol Bioprocess Eng 27(4):586–595. https://doi.org/10.1007/s12257-022-0065-1
Restrepo-Flórez J-M, Bassi A, Thompson MR (2014) Microbial degradation and deterioration of polyethylene – a review. Int Biodeterior Biodegr 88:83–90. https://doi.org/10.1016/j.ibiod.2013.12.014
Rochman CM, Browne MA, Underwood AJ, Van Franeker JA, Thompson RC, Amaral-Zettler LA (2016) The ecological impacts of marine debris: unraveling the demonstrated evidence from what is perceived. Ecology 97(2):302–312
Roy PK, Titus S, Surekha P, Tulsi E, Deshmukh C, Rajagopal C (2008) Degradation of abiotically aged LDPE films containing pro-oxidant by bacterial consortium. Polym Degrad Stab 93(10):1917–1922. https://doi.org/10.1016/j.polymdegradstab.2008.07.016
Sanluis-Verdes A, Colomer-Vidal P, Rodriguez-Ventura F, Bello-Villarino M, Spinola-Amilibia M, Ruiz-Lopez E, Illanes-Vicioso R, Castroviejo P, Aiese Cigliano R, Montoya M, Falabella P, Pesquera C, Gonzalez-Legarreta L, Arias-Palomo E, Solà M, Torroba T, Arias CF, Bertocchini F (2022) Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. Nat Commun 13(1):5568. https://doi.org/10.1038/s41467-022-33127-w
Seong HJ, Yao Z, Jang Y-S (2023) Complete genome sequence of Acinetobacter nosocomialis GNU001, isolated from a plastic-containing landfill. Microbiol Resour Announc 12(1):e01077-e1122. https://doi.org/10.1128/mra.01077-22
Singh M, Lee KE, Vinayagam R, Kang SG (2021) Antioxidant and antibacterial profiling of pomegranate-pericarp extract functionalized-zinc oxide nanocomposite. Biotechnol Bioprocess Eng 26(5):728–737. https://doi.org/10.1007/s12257-021-0211-1
Sridharan R, Krishnaswamy VG, Kumar PS (2021) Analysis and microbial degradation of low-density polyethylene (LDPE) in Winogradsky column. Environ Res 201:111646. https://doi.org/10.1016/j.envres.2021.111646
Tribedi P, Sil AK (2013) Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ Sci Pollut Res 20(6):4146–4153. https://doi.org/10.1007/s11356-012-1378-y
Vimala PP, Mathew L (2016) Biodegradation of polyethylene using Bacillus Subtilis. Procedia Manuf 24:232–239. https://doi.org/10.1016/j.protcy.2016.05.031
Volke-Sepúlveda T, Saucedo-Castañeda G, Gutiérrez-Rojas M, Manzur A, Favela-Torres E (2002) Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J Appl Polym Sci 83(2):305–314
Wei R, Zimmermann W (2017) Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb Biotechnol 10(6):1308–1322
Woo JE, Seong HJ, Lee SY, Jang Y-S (2019) Metabolic engineering of Escherichia coli for the production of hyaluronic acid from glucose and galactose. Front Bioeng Biotechnol 7:351. https://doi.org/10.3389/fbioe.2019.00351
Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD (2011) Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environ Health Perspect 119(7):989–996
Yang J, Yang Y, Wu W-M, Zhao J, Jiang L (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol Lett 48(23):13776–13784
Yao Z, Seong HJ, Jang Y-S (2022a) Degradation of low density polyethylene by Bacillus species. Appl Biol Chem 65(1):84. https://doi.org/10.1186/s13765-022-00753-3
Yao Z, Seong HJ, Jang Y-S (2022b) Environmental toxicity and decomposition of polyethylene. Ecotoxicol Environ Saf 242:113933. https://doi.org/10.1016/j.ecoenv.2022.113933
Yin C-F, Xu Y, Zhou N-Y (2020) Biodegradation of polyethylene mulching films by a co-culture of Acinetobacter sp. strain NyZ450 and Bacillus sp. strain NyZ451 isolated from Tenebrio molitor larvae. Int Biodeterior Biodegr 155:105089. https://doi.org/10.1016/j.ibiod.2020.105089
Yoon YR, Jang Y-S (2021) Potential of Baeyer-Villiger monooxygenases as an enzyme for polyethylene decomposition. J Appl Biol Chem 64(4):433–438
Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K (2016) A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 351(6278):1196–1199
Zahra S, Abbas SS, Mahsa M-T, Mohsen N (2010) Biodegradation of low-density polyethylene (LDPE) by isolated fungi in solid waste medium. Waste Manage 30(3):396–401. https://doi.org/10.1016/j.wasman.2009.09.027
Zhu Y-J, Olson N, Beebe TP (2001) Surface chemical characterization of 2.5-μm particulates (PM2. 5) from air pollution in salt lake city using TOF-SIMS, XPS, and FTIR. Environ Sci Technol 35(15):3113–3121
Acknowledgements
We extend our sincere gratitude to the **ju Landfill Office (**ju, Korea) for their support in providing the necessary resources, space, and information for the isolation of PE-degrading microorganisms.
Funding
This study was supported by the Technology Development Program for the Decomposition of Agricultural Polyethylene Waste using Systems Metabolic Engineering for Microorganisms, funded by the Cooperative Research Program for Agricultural Science & Technology Development, Rural Development Administration, Republic of Korea (Project Nos. PJ01492601 and PJ01492603).
Author information
Authors and Affiliations
Contributions
YJK, NJK, and YSJ conceived the project. HJS, YJK, HK, and SBB performed experiments. HJS, YJK, ZY, NJK, and YSJ analyzed data. HJS and YSJ wrote the manuscript. YJK and NJK revised the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seong, H.J., Kim, H., Ko, YJ. et al. Enhancing polyethylene degradation: a novel bioprocess approach using Acinetobacter nosocomialis pseudo-resting cells. Appl Microbiol Biotechnol 108, 86 (2024). https://doi.org/10.1007/s00253-023-12930-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12930-5