Abstract
Cornus mas L. (Cornelian cherry, CM) fruits were dehydrated by solar-drying (SD) and freeze-drying (FD), and in addition to sugar and mineral contents, the free and insoluble-bound phenolics were determined in fresh and dried fruits. After subjecting the sample to simulated in vitro digestion, the change of free and bound phenolics at gastric and intestinal digestion steps was evaluated in fresh and dried CM fruits. In fresh CM fruits, the total phenolic content (TPC) was dominated by the bound fraction, whereas the contribution of free phenolics to the total content (free + bound) became more dominant (731–1439 mg GAE/100 g dw) in the dried fruits. The bioaccessibility (BI%) of TPC from fresh CM after digestion was 193%, whereas it was 18.60 and 48.02% for SD and FD fruits, respectively. The contribution free fraction to the total TPC value was around 28% in nondigested fresh samples and increased to 94% in digested samples; however, in dried samples, it was 64% prior to digestion and only increased to 70% in digested samples. A total of 17 phenolic compounds were identified in CM fruits: chlorogenic acid, caffeic acid, epicatechin, quercetin, cyanidin-3-O-glucoside, and pelargonidin 3-O-glucoside were only detected in the free fraction; gallic acid, vanillic acid, ferulic acid, and kaempferol were detected in higher amounts in the bound fraction. The quantity of detected phenolics in the nondigested sample generally decreased from the gastric to the intestinal stage of digestion. The release of phenolics from the fruit matrix and their degradation occurred simultaneously during digestion, and this could be affected by the state of the fruit, e.g., fresh or dried.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cornelian cherry, also known as Cornus mas L. (CM), is a plant belonging to the genus Cornus, a family member of the Cornaceae. CM is widely grown in Turkey, Romania, Bulgaria, Italy, Southern Europe, and Southwest Asia. CM has been known since ancient times, and it is used in folk medicine purposes mostly for diarrhea, hemorrhoids, diabetes, sore throat, digestive ailments, measles, chicken pox, anemia, rickets, liver, and renal disease. The health-promoting properties of CM have been associated with the bioactive components. Studies have reported that CM contains many bioactive components, such as polyphenols, vitamins, and minerals [1, 2]. In addition, it has been found that polyphenols and anthocyanins (which give an attractive red color to fruits) contribute substantially to the antioxidant intake [3]. Therefore, CM is a promising functional fruit due to its biological activities, such as antioxidant, anti-inflammatory, antiobesity, antidiabetic, antiatherosclerosis, and hypolipidemic [1, 2, 4,5,6,7,8].
CM is generally consumed fresh but also processed in the form of juice, syrups, gels, jams, and other traditional products, as well as alcoholic beverages like liquor, wine, and beer [1, 9]. Fresh CM rapidly deteriorates and is perishable, and the fruit is not available all year round due to the short harvest season, which causes limited commercialization and consumption [2]. Drying is one of the oldest food preservation methods used to extend the shelf life of fruits. Depending on the method employed, the physicochemical properties of dried fruits, such as phenolic content, sugar composition, rehydration capacity, color, and morphological properties. Solar dying (SD), which can be used for open sun-drying, white-cloth shading, and black-cloth shading, has a lower cost, but the fruits were exposed to oxygen and high temperatures for an extended period [10]. On the other hand, freeze-drying (FD) is considered the best method that operates at low temperatures under vacuum, which contributes to minimizing the degradation of organic components and sensorial properties, but it is expensive [11, 12].
Although the previous research investigated the phenolic contents of dried and fresh CM fruit and the in vitro bioaccessibility of phenolics from fresh CM fruits [13, 14], those studies mainly focused on extractable (free) phenolics. However, bound phenolics that cannot be extracted by only organic solvents play an important role in the changes of individual phenolic contents during in vitro gastrointestinal digestion. Because the chemical bonds that attach phenolics to the cell wall components can be broken by hydrolysis with acid, alkaline, or enzymatic solutions during digestion, those phenolics could be released, be available for absorption, and alter the antioxidant properties of the digested fruit. This study aims to provide a novel perspective on the in vitro digestibility profile of fresh and dried CM fruits, which has not been previously explored in the literature. Therefore, this study evaluates the effects of different drying methods (solar drying-SD, and freeze drying-FD) on the physicochemical properties, phenolic compounds, and antioxidant capacities of CM fruits. İn addition, the effects of in vitro digestion on free and bound phenolics and the antioxidant capacity of fresh, SD, and FD CM fruits were investigated.
Materials and methods
Chemicals
Folin–Ciocalteu’s Phenol Reagent (2 N), gallic acid, sodium carbonate (Na2CO3), sodium nitrite (NaNO2), aluminum chloride, 6-hydroxy-2,5,7,8-tetramethylchroman-2carboxylic acid (Trolox, 97%), 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS > 98%) and 2,2-diphenyl-1- picrylhydrazyl (DPPH, 95%), tripyridyltriazine (TPTZ), ferrous chloride, neocuproine, α-amylase (A1031), pepsin (P7012), pancreatin (P7545) and bile salt (B8631) phenolic standards, hydrochloric acid, methanol (> 99% purity) and acetonitrile (> 99% purity) for high-performance liquid chromatography (HPLC analysis) were obtained from Sigma-Aldrich LLC. (Steinheim, Germany). They were all stored according to the instructions of the producers, such as enzymes, and standards were all stored at – 20 °C. The salts and solvents were stored in a dry, well-ventilated area, away from direct sunlight, at room temperature.
CM fruits and drying procedure
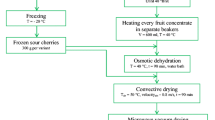
Fresh CM was obtained from Erzurum, Gölbaşı (Turkey) during harvesting time in August–September 2020 and stored at 4 °C until the drying process. The analysis of whether the purchased fruits belong to the "Cornus mas L." family was carried out with the MARE number analyzed by the previous study [4]. Fresh CM fruits were dried by two different methods (Fig. 1): SD, and FD. The fresh and dried CM samples were stored at -20 °C until analysis. Each drying experiment was conducted in triplicate. The moisture contents of samples were 75.12 ± 1.99% (fresh), 16.54 ± 1.25% (SD), and 11.87 ± 0.91% (FD).
Determination of color, ascorbic acid, and mineral content
The color of fresh and dried CM samples was measured using a chromameter (Konica Minolta CR400 Tokyo, Japan) and expressed as L* (whiteness/darkness), a* (redness/greenness), and b* (yellowness/blueness) values. The colorimeter was calibrated to a white calibration plate (CR-A44 Konica Minolta Sensing Inc., Osaka, Japan) before use. The ascorbic acid was determined by the modified 2,6-dichlorophenol indophenol dye method as described by Cemeroglu [15]. The mineral content of the samples was determined with an inductively coupled plasma-optical-emission spectrometer (ICP-OES) (Shimadzu-ICPE-9000, Japan) according to da Silva Haas et al. [16]. The sample was digested in a microwave oven with a mixture of 7.5 mL HNO3/2.5 mL H2O2. Argon (99.95%) was the main, auxiliary, and the nebulizer gas. The operating parameters were 1200 W, and the nebulizer and auxiliary gas flow rates were 0.70 and 0.20 L/min. The calibration was done using external calibration with standard solutions in the concentration range of 0 to 200 μg/L for elements. The calibration standards were prepared by dilution of multielement standard solution IV (Supelco, Merck) at 1 mg/mL. The analysis was performed in duplicate. The results were given as mg/100 g fresh weight (fw).
Extraction of phenolic fractions
Free and insoluble-bound phenolic compounds from the fresh and dried CM fruits were extracted and fractionated according to the method described by Arruda et al. [17] and Yu et al. [18] with slight modifications. The flowchart of the extraction procedure was given in Fig. 1. Based on our preliminary results yielding the highest phenolic content, the extraction of phenolic fractions was set as follows: The samples were mixed with 80% MeOH (1% HCl) (1:15, w/v) and homogenized with a T-25 Ultra-Turrax (Ika Labortechnik, Stauden, Germany) at 10 000 rpm for 5 min, and kept in an ultrasonication bath (Daihan Scientific, WUC-A03H, Korea) for 0.5 h at room temperature. The supernatant was separated by centrifugation (2480 × g, 10 min, 4 ºC), and the residue was re-extracted three times at the same conditions. The combined supernatants were evaporated to dryness and dissolved in methanol (5 mL) for the determination of free phenolics. The solid residue obtained after the extraction of the free phenolic was hydrolyzed with 4 M NaOH (10 mM EDTA, 1% ascorbic acid) 1:20 (w/v) for 4 h at room temperature using a water bath shaker (150 rpm) to release insoluble-bound phenolics. The pH of the mixture was adjusted to 2 using 6 M HCl and centrifuged at 2480 × g for 5 min at 4 °C. Then, the supernatant was extracted three times with an equal volume of hexane to remove interfering lipids. The aqueous phase was extracted three times with diethyl ether-ethyl acetate (1:1, v/v) at a ratio of 1:1 (v/v). The organic phases were combined and filtered through anhydrous sodium sulphate (No. 1 Whatman filter paper) and evaporated to dryness under vacuum at 35 °C and dissolved in methanol for the analysis of the insoluble-bound phenolic fraction.
Total phenolic, total flavonoid, and total anthocyanin content
The total phenolic content (TPC) [19], the total flavonoid content (TFC) [20], and the total monomeric anthocyanin (TAC) content [21] were determined. The absorbances were measured at 760 nm, 510 nm, and 517 nm for TPC, TFC, and TAC, respectively, using a Shimadzu 150 UV-1800 spectrophotometer (Kyoto, Japan). The results of TPC, TFC, and TAC were given as mg gallic acid equivalent (GAE), mg catechin equivalent (CE), and mg cyanidin-3-glucoside (cyn3glu) /100 g dry weight (dw), respectively. The linear range of the gallic and catechin standard curves was from 0.01 to 0.15 mg/mL and 0.01 to 0.4 mg/mL, respectively (r2 = 0.99).
Antioxidant capacity assays
DPPH radical scavenging activity [22], the copper-reducing antioxidant capacity (CUPRAC) [23], ABTS radical scavenging activity [24], and the FRAP assay [25] were performed, and the absorbances were measured at 517 nm, 450 nm, 734 nm, and 593 nm for DPPH, CUPRAC, ABTS, and FRAP, respectively. A blank test tube without extracts, but with solvent was also subjected to analysis. Their results were expressed as μmol Trolox equivalent (TE)/g dw, and the curve for the Trolox was linear in the concentration range of 100–1998 µM, 40–799 µM, 10–799 µM for DPPH with CUPRAC, ABTS, and FRAP, respectively (r2 = 0.99).
HPLC analysis of phenolics and sugar content
The individual phenolics and anthocyanins were evaluated using an HPLC system (LC-20AD pump, SIL-20A HT autosampler, CTO-10ASVP oven, DGU-20A5R degasser, CMB-20 A communication module) coupled to a diode array detector, SPDM20A DAD (Shimadzu Corp., Kyoto, Japan), according to Ozcan et al. [26]. The retention times and external standard curves were used to perform identification and quantitative analyses. A 0.45-µm membrane filter was used to filter the extracts. Separations were conducted at 40 °C on a reversed-phase Intersil ODS C-18 column (250 mm × 4.6 mm length, 5 μm particle size, GL Sciences, Tokyo, Japan). The mobile phase included solvent A, Milli-Q water with 0.1% v:v trifluoroacetic acid (TFA), and solvent B, acetonitrile with 0.1% v:v TFA. Linear gradient elution was used as follows: at 0 min 95% of solvent A, at 50 min 65% of solvent A, at 52 min 25% of solvent A, at 59 min returns to initial conditions. The flow rate was 1 mL/min. Chromatograms were recorded at 254, 265, 278, 280, 291, 295, 310, 322, 325, 367, and 517 to quantify phenolic acids, flavonoids, and anthocyanins. The samples were analyzed for their sugar (glucose, fructose, and sucrose) composition. The samples were immersed in distilled water (1:10, w: v) and homogenized with a T-25 Ultra-Turrax (Ika Labortechnik, Stauden, Germany) at 10,000 rpm for 5 min. The mixtures were kept in an ultrasonication bath (Daihan Scientific, WUC-A03H, Korea) for 0.5 h at room temperature, the supernatant was separated, and the residue was re-extracted at the same conditions. The supernatants were combined and filtered. The filtrate was concentrated by a rotary evaporator at 65 °C until its weight was 10% less than the weight of the original filtrate [27]. All extracts were stored at -20 °C in the dark until use. The sugar composition was analyzed on the same HPLC model and RID-20A refractive index detector (RID) system. The mobile phase was acetonitrile: water (83:17, v/v) with a flow rate of 2 mL/min. A 10 µL of filtered (0.45 µm) sample was injected into the Agilent Zorbax NH2 column (5 µm, 4.6 × 250 mm). The stock solutions of reference standards were prepared in methanol (for phenolics) and ultrapure water (for sugars). Working solutions were prepared by diluting the stock solutions with the appropriate solvent to have 10–100 µg/mL and 75–20,000 µg/mL for phenolic and sugar standards, respectively). Calibration curves were generated by graphing the peak areas of the identified compounds relative to the peak areas against the concentration of the standard compound. The calibration curves based on triplicate injections demonstrated good linearity, with R2 values exceeding 0.99 (peak area vs concentration). All analyses were performed in triplicates and the results were given as mg/100 g dw.
In vitro simulated digestion process
In vitro simulated digestion was performed according to Yu et al. [18], and Brodkorb et al. [28] (Fig. 1). 2.5 g of samples were mixed 1:1 (w: v) with simulated salivary fluid (SSF), α-amylase (75 U/mL), CaCl2 (0.75 mM), and vortexed for 2 min at 37 °C (pH 7.0). The oral bolus was diluted with simulated gastric fluid (SGF) (1:1, v: v), CaCl2 (0.075 mM), pepsin (2000 U/mL) and incubated for 2 h at 37 °C (pH 3.0). The gastric chyme was diluted with simulated intestinal fluid SIF (1:1, v:v), CaCl2 (0.3 mM), pancreatin (100 U/mL), and fresh bile (10 mM) and incubated for 2 h at 37 °C (pH 7.0). The formulations of the salivary, gastric, and intestinal fluids are described in Table S1. After each stage of digestion, the tubes consisted of digestion medium and samples were centrifuged at 2480 × g for 10 min at 4 °C, filtered (0.45 μm), immediately frozen in liquid nitrogen and lyophilized, and dissolved in methanol for the analysis of free phenolic fraction. The solid residues that remained in the tubes at the end of gastric and intestinal digestion were extracted according to the method described in 2.4 for the determination of insoluble-bound phenolic fraction. A blank test tube without samples but with all digestion fluids was also subjected to analysis.
This bioaccessibility index was determined as follows:
A1 and A0 are the amount of free phenolics determined in the intestinal phase after digestion and the amount of free phenolics in the nondigested sample, respectively.
Statistical analysis
All experiments were carried out in triplicate from at least duplicated samples, and the data were reported as the mean ± standard deviation. Statistical analysis was done by SPSS (IBM version 20, USA). An one-way ANOVA followed by Tukey’s posthoc test was applied to evaluate the differences among the fresh and dried CM samples. The differences in the free, bound, and total phenolic values between the gastric and intestinal phases of each digested sample were evaluated by a T test.
Results and discussion
The sugar, ascorbic acid, mineral content, and color of fresh and dried Cornus mas (CM)
The physicochemical properties of fresh and dried CM fruits are given in Table 1. The moisture content of fresh fruit was 75.12 ± 1.99% and reduced to 11.87 ± 0.91 and 16.54 ± 1.25% by SD and FD, respectively. Glucose and fructose were the main monosaccharides detected in fresh and dried fruits [9]. Although the level of fructose did not differ among samples, as compared to fresh fruit, there was a slight decrease in the fructose, and the reduction of glucose was only significant in SD fruit. Similarly, the decrease in the sucrose was determined in the SD fruit, but not in the FD sample. Due to exposure to heat for a relatively longer time, those sugars might be involved in nonenzymatic browning and caramelization reactions [29]. In addition, the hydrolysis of sucrose to its monosaccharides may occur due to the conditions of SD, which can explain the greater degree of reduction as compared to FD and fresh samples [30, 31].
The total sugar content of fresh and dried fruits ranged from 40.33 to 49.02 g/100 g dw (equivalent to 142.82 g/kg fw, 392.34 g/kg fw, and 397.55 g/kg fw for fresh, SD, FD, and CM fruits, respectively), and our results were comparable to the findings of previous studies. Tural & Koca [32] reported that the mean total sugar content was 93.42 ± 18.67 g/kg in twenty-four CM fruits collected from Samsun, Turkey, whereas it varied from 40.6 to 61.70 g/kg in the study by Ozrenk et al. [33] conducted in Erzincan, Turkey. The sugar content of fruits can be affected by various factors, such as the genotype, origin, and ripeness of the fruits. The ascorbic acid content of fresh fruit was 317.78 ± 4.07 mg/100 g dw, comparable to the findings of Rosu et al. [34] (419.08 ± 72.59 mg/100 g dw), and in the range of 0.11 and 1.06 mg/g fw reported by previous studies in Turkey [35,36,37,38]. The ascorbic acid content of CM fruits was substantially lost by drying, and as expected [11, 12], the reduction was less in the samples dried by FD (82.93 ± 1.11 mg/100 g dw) compared to SD samples (23.99 ± 0.13 mg/100 g dw).
The lightness (L value) and redness (a value) of FD fruits (34.53 ± 0.87 and 39.57 ± 0.78) were higher than those of fresh and SD fruits (30.42–30.85 and 28.85–12.90). The b value of fresh CM (– 3.22 ± 0.49) has a negative value with more bluish tints, and it was slightly reduced in FD fruit (– 2.76 ± 0.36) and did not show any significant difference in SD fruit (– 3.60 ± 0.15). The change in color values in dried fruits compared with fresh samples might be related to the textural change as well. For example, the presence of air voids and pores and the replacement of free water with air would occur by FD; thus, light diffusion through the sample would be altered [39]. Although the lower redness (a) value of SD fruits might be related to enzymatic browning reactions and degradation of anthocyanin components due to exposure to air temperature and oxygen during sun-drying. Similar to our results, Szczepaniak et al. [40] reported that when compared with fresh CM fruits, higher L and lower a and b values were determined in frozen samples.
CM fruits and leaves are reported to have high levels of minerals [2]. Studies reported that the fruit of CM was rich in K, P, Ca, Mg, Cu, Fe, Mn, and Zn [38, 41, 42]. According to our findings, among minerals, K content was the highest, followed by Na, Mg, and Ca (Table 1). The minerals are heat-stable; they will not be expected to be degraded during any heat treatment. Compared to fresh CM, the content of minerals was increased in dried fruits as a result of concentration, K and Mg were higher in FD fruit, whereas Na was detected in higher amounts in SD fruits, and the level of Ca was not statistically different between dried fruits. The minerals in plants were present as structural elements of proteins, amino acids, enzymes, pigments, and cell wall constituents rather than free ions. Therefore, the content of tannins and other polyphenols, the depolymerization of cell wall components, the change in insoluble dietary fiber content, and the cell structure attained after drying could influence the leaching, solubility, and binding of minerals with other components [29, 43].
Total phenol (TPC), total flavonoid (TFC), total anthocyanins content (TAC), antioxidant capacity, and individual phenolics of fresh and dried Cornus mas (CM)
The TPC, TFC, TAC, antioxidant capacities, and individual phenolics and anthocyanins of fresh and dried CM fruits are presented in Figs. 2, 3, and Table 2. The TPC of FD fruit (2259.43 ± 8.51 mg GAE/100 g dw) was higher than that of fresh (1587.57 ± 12.52 mg GAE/100 g dw) and SD fruits (1145.72 ± 7.54 mg GAE/100 g dw). Thus, the drying processes had a statistical impact on the TPC (p < 0.05). As expected, the TPC of SD fruits was the lowest among samples, both due to the possible activity of polyphenol oxidase and exposure to air temperature [44]. A similar trend was observed for TFC and TAC, and FD fruits provided the highest value, whereas SD fruit had the lowest content, and the results were statistically significant (p < 0.05).
The total phenolic (TPC), flavonoid (TFC), and anthocyanin contents (TAC) of fresh, SD and FD CM samples under three conditions [before digestion (ND) and after gastric (SGF) and intestinal (SIF) digestion], including free and bound fractios. SD solar died, FD freeze-fried, CE catechin equivalent, GAE gallic acid Equivalent, cyn3glu cyaniding-3-glucosidem, dw dry weight. Means with different superscript letters (X–Z) among sample (fresh, SD, and FD fruits) before digestion (ND) are significantly different (p < 0.05) for total content (free + bound). Means with different superscript letters (d-e) are significantly different (p < 0.05) between the total content (free + bound) of each sample between gastric and intestinal digestion steps
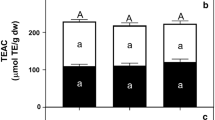
Total antioxidant capacities of fresh, SD, and FD CM samples under three conditions [before digestion (ND) and after gastric (SGF) and intestinal (SIF) digestion], including free and bound fractions. SD solar dried, FD freeze-dried, TE Trolox equivalent, dw dry weight. Means with different superscript letters (X–Z) among samples (fresh, SD, and FD fruits) before digestion (ND) are significantly different (p < 0.05) for total content (free + bound). Means with different superscript letters (d-e) are significantly different (p < 0.05) between total content (free + bound) of each sample between gastric and intestinal digestion steps
The increase in measured phenolics in FD fruits as compared to fresh samples could be associated with the elevated extractability of those compounds into the extraction solvent due to the mild microstructural changes induced by the ice-crystal formation during FD, and the higher rehydration of FD with the solvent due to the porous structure obtained [45, 46]. The TPC and TFC values included free and insoluble-bound phenolics and flavonoids. In fresh CM fruits, the total phenolic content was dominated by the bound fraction [47, 48], whereas its contribution to the total content (free + bound) was altered by drying. The TPC and TFC values of the free fractions of both dried fruits were higher than those of fresh samples, and the contribution of free phenolics became more dominant in the dried samples (Fig. 2). For example, in a fresh sample, around 70% of total TPC was contributed by the bound fraction, whereas in SD and FD fruit, the contribution of the bound fraction to total TPC was around 36%. This could be related to the weakened attachment of bound phenolics to the cell walls due to drying, and bound phenolics became more available in the free fraction of the extract [49, 50].
The TAC value of fresh CM (123.30 ± 2.71 mg cyn3glu/100 g dw) was comparable with the amount (111–136.63 mg cyn3glu/100 g dw) determined in previous studies for different CM genotypes [9, 40]. The loss of TAC in SD fruit was substantial (5.50 ± 0.11 mg cyn3glu/100 g dw). It is known that the magnitude and duration of temperature have a strong influence on anthocyanin instability, and several studies reported a logarithmic course of anthocyanin destruction with an arithmetic increase in drying temperature [29]. The analysis of bound fraction involves alkali extraction, and due to the instability of anthocyanins, most probably as a result of the flavylium cation hydrating to form the colorless carbinol or pseudobase, and then turning into the yellow-colored chalcone. Therefore, anthocyanins might not be determined in the bound phenolic fraction.
Phenolic compounds exhibit a variety of functions, primarily due to their antioxidant activities acting as radical quenchers, reducing agents, transition metal chelators, etc. [18]. When considering the difference in antioxidant mechanisms, the antioxidant capacity of CM fruits was determined by different assays. The ABTS, CUPRAC, DPPH, and FRAP antioxidant capacity values of fresh CM were 208.66, 87.07, 130.45, and 114.23 µmol TE/g dw (Fig. 3), respectively. In the study of Martinović & Cavoski [51], the DPPH values of different CM cultivars ranged from 6.23 to 19.03 µmol TE/g fw, the ABTS value varied from 4.41 to 14.75 µmol TE/g fw, and the FRAP value differed from 15.09 to 59.54 µmol Fe2+/g fw. Except for DPPH, in which the fresh and FD samples provided similar values, the antioxidant capacity (total + free phenolics) values of FD fruits were higher than those of the SD fruit and fresh sample, and as expected, SD resulted in a reduction of antioxidant capacity values. In fresh fruits, the antioxidant capacities of free fractions determined by all assays were lower than those of insoluble-bound fractions. Whereas in dried samples, the contribution of free and bound phenolics to antioxidant capacity values depended on both the assay and the method of drying. Compared to the bound fraction, the free fraction of FD fruit yielded higher ABTS and DPPH values but lower FRAP values. On the contrary, the free fraction of SD fruit yielded lower DPPH values as compared to the bound fraction, whereas FRAP and ABTS values were similar in both fractions.
A total of 17 phenolic compounds were identified in CM fruits (Table 2). Among phenolic acids, the highest content belonged to gallic acid, 3-hydroxybenzoic acid, and among flavonols, epicatechin, quercetin-3-galactoside, and quercetin-3-rutinoside was identified at high concentrations in fresh CM. The presence of gallic acid, chlorogenic acid, hydroxybenzoic acids, rutin [52, 53], quercetin-3-galactoside, kaempferol 3-O-glucoside [54], ellagic acid, and its derivatives in CM fruits have been reported previously [53]. Chlorogenic acid, caffeic acid, epicatechin, and quercetin were only detected in the free fraction of the samples. Compared to the free fraction, only gallic acid, vanillic acid, ferulic acid, and kaempferol were detected in higher amounts in the bound fraction. Similarly, Chen et al. [55] reported that kaempferol, ferulic, and sinapic acids were highly found in the bound-phenolic fractions of cranberry beans.
Compared with fresh fruits, the chlorogenic acid content in dried CM fruits is accompanied by increased caffeic acid and neochlorogenic acid content (Table 2). Chlorogenic acid is an ester of caffeic and quinic acids, and its molecular structure contains ester bonds and unsaturated double bonds, which are unstable and easily oxidized. In addition, chlorogenic acid can also undergo isomerization reactions, yielding neochlorogenic and cryptochlorogenic acid [56], and compared to fresh fruits, the neochlorogenic acid content of dried fruits was higher in dried CM fruits. The increased level of ellagic acid in the free fraction of dried fruits could also be the result of the depolymerization of some ellagitanins present in the plant structure. Similarly, epicatechin and catechin were the basic units of condensed tannins, mainly identified in the free fraction [57], and their amount was increased in dried samples.
The major anthocyanins detected in fresh CM fruit were cyanidin 3-O-glucoside (36.21 ± 0.57 mg/100 g dw), pelargonidin 3-O-glucoside (33.97 ± 0.05 mg/100 g dw), and the presence of cyanidin 3-O-rutinoside (0.95 ± 0.02 mg/100 g dw) and peonidin 3-O-glucoside (0.56 ± 0.01 mg/100 g dw) were also quantified. The detected anthocyanin content was substantially lower in dried samples (Table 2). Similarly, the level of all individual anthocyanins was decreased by oven-drying, and the results were statistically significant (p < 0.05), whereas in FD grapes and grape skins, only some individual anthocyanin contents were reduced [12, 29]. As expected, anthocyanins were only detected in the free fraction because the alkaline hydrolysis employed during the extraction of bound phenolics would be detrimental to any remaining anthocyanins.
Effect of drying methods on the in vitro bioaccessibility of Cornus mas phenolics and antioxidant capacity
The bioaccessibility of a phytochemical is the fraction released from the food matrix during digestion in a form accessible for absorption in the small intestine or biotransformed by the gut microbiota. It is impacted by many factors, including the nature of phytochemicals, the initial condition of food, and the process occurring inside the gastrointestinal tract. In this study, the fresh and dried CM fruits were subjected to in vitro oral, gastric, and intestinal digestion conditions consecutively. We determined the amounts of TPC, TFC, TAC, antioxidants, and individual phenolics in both the digestive fluids (as the free fraction) and the solid residue that remained in the test tube after each digestion step. This residue was extracted using the same method applied to measure the insoluble phenolics in nondigested samples. The results are given in Figs. 2 and 3.
TPC (free + bound) of the fresh fruit (1587 mg GAE/100 g dw) was first decreased to 700 mg GAE/100 g dw by gastric digestion and increased to 920 mg GAE/100 g dw in the following intestinal step. A similar trend, decreasing by gastric digestion and increasing at the intestinal step, was reported by Chait et al. [58] and Seke et al. [59]. Although the majority of total phenolics were comprised of the bound fraction (72%) in the nondigested sample, in digested fruit, the majority of phenolics were derived from the free fraction (94%). Therefore, the bioaccessibility of TPC from fresh fruit after digestion reached 193% of the initial free phenolic fraction. The increase in the amount of free phenolic compounds after digestion has been confirmed in studies such as vegetable juices [60], nutmeg [61], and persimmon fruit [62].
TFC of the fresh fruit also showed a similar trend, decreasing to 73 mg CE/100 g dw during the gastric stage and increasing to 124 mg CE/100 g dw during intestinal digestion. Bound fractions made up 61% of the TFC of the nondigested sample, while the proportion of free and bound fractions remained similar throughout the digestion steps. The bioaccessibility of TFC after digestion reached 51.13% of the initial free fraction of fresh fruit. The intestinal digestive enzymes and bile salts that act on the food matrix could facilitate the passage of bound phenolic compounds into the digestive juices [63]. The release of phenolics from the matrix and their degradation could occur simultaneously during digestion, and this could be affected by the state of the fruit, drying method, etc.
TPC (free + bound) and TFC (free + bound) of SD fruit were increased to 1429 GAE/100 g dw and 252 CE/ 100 g dw by the gastric stage and decreased to 194 GAE/100 g dw and 53 CE/100 g dw in the intestinal stage, whereas TPC (free + bound) and TFC (free + bound) of FD fruit were decreased both in the gastric and intestinal stages (Fig. 2). It is known that the FC reagent used in the TPC assay could also give positive results with the Maillard reaction products [64]. During SD, invertase and organic acids released due to the disruption of the cell membrane disruption [65] may hydrolyze the sucrose of the CM fruit to the reducing sugars involved in Maillard browning reactions. Therefore, SD samples that might undergo the Maillard reaction could present higher TPC values during gastric digestion.
The contribution of the free fraction to the total TPC value was around 64% in nondigested dried samples, reduced to around 40% after gastric digestion, and increased up to 70% after intestinal digestion. The bioaccessibility index of TPC based on the free phenolic fraction of digested and nondigested samples was calculated as 18.60 and 48.02% for SD and FD fruit, respectively. Adom & Liu reported that insoluble-bound phenolics can reach the large intestine by resisting gastric and intestinal digestion due to the difficult digestion of cell wall components [66]. If the phenolic compounds are closely bound to the cell matrix, they are less affected by the neutral pH environment and enzymes in the small intestine [67], which could explain why SD samples compared to fresh and FD fruits yielded lower phenolic values after digestion.
After the gastric and intestinal phases, there was a decrease in the TACs of all samples. After intestinal digestion, the TAC of fresh CM was found to be 23 mg cyn3glu/100 g dw, and the BI % of TAC was changed from 3.41 (FD) to 10.22 (fresh CM). The results were statistically significant (p < 0.05). David et al. [13] reported that after intestine digestion, the TAC of fresh CM fruit was found to be 26.46 mg cyanidin-3-O-glucoside /L TAC (free + bound) of the SD and FD samples also followed a similar trend; TAC for SD fruit (5 mg cyn3glu/100 g dw) was decreased to 0.5 mg cyn3glu /100 g dw by the gastric stage and exhausted in the intestinal digestion. TAC for FD fruits (196 mg cyn3glu/100 g dw) was decreased to 21 mg cyn3glu /100 g dw, and 6 mg cyn3glu /100 g dw by gastric and intestinal steps, respectively. Ozkan et al. [29] also reported that simulated intestinal digestion had a dramatic impact on the anthocyanins in fresh and dried grape samples.
In the gastric phase, the total ABTS, DPPH, and FRAP values of fresh and dried CM samples decreased, while the CUPRAC value increased. Additionally, after the gastric phase, the free fractions of ABTS, CUPRAC, and FRAP assays increased for fresh CM fruit (Fig. 3). Lee et al. [68] reported that after the gastric phase, total ABTS and FRAP values increased for fresh and oven-dried omija, while ABTS, CUPRAC, and FRAP values decreased for FD-omija. Moreover, phenolics may combine with trypsin to form a complex, which reduces its solubility and significantly inhibits its electron-giving ability, thus reducing its antioxidant ability [18].
In all CM samples except the FRAP value for FD fruits, the antioxidant capacity of the intestinal phase was lower than the gastric phase. The condensation and polymerization reactions of phenolics in the intestinal environment might produce substances with a higher molecular weight that would impair their diffusion and solubility through the membrane [29]. After the intestinal phase, the total ABTS, CUPRAC, DPPH, and FRAP values of fresh CM were found as 65, 85, 22, and 58 µmol TE/g dw, respectively. These values were 59, 44, 14, and 46 µmol TE/g dw for SD fruits, and 134, 120, 16, and 87 µmol TE/g dw for FD fruits, respectively. A similar trend (decrease of the antioxidant capacity during gastrointestinal digestion) has been reported in the literature, for instance, ABTS, CUPRAC, DPPH, and FRAP of different fruits were observed to be significantly decreased after the digestion process [29, 69,70,71]. Polyphenols are extremely sensitive to alkaline conditions. Alkaline pH degrades antioxidants following the pancreatic digestion phase, resulting in an overall reduction of antioxidant capacity after in vitro digestion [71, 72].
Even though the initial TPC, TFC, TAC, and antioxidant capacities were higher in the FD, the bioaccessibility indices were not always highest for the FD (Fig. 4). In our study, the BI % of TPC was changed from18.60% (SD) to 193.63% (fresh CM), whereas the BI % of TAC was changed from 10.22% (fresh CM) to 3.41% (FD). In the study of Ozkan et al. [29], after the simulated digestion of the Isabel grape, the BI % of TPC in fresh and FD samples was found to be 33.04 and 44.98%, while the BI% of TAC in fresh and FD samples was found to be 1.49 and 0.77% respectively. In our study, the BI % of ABTS ranged from 23.50% (FD) to 97.49% (fresh CM), while the BI % of FRAP ranged from 37.59% (FD) to 70.12% (fresh CM) (Fig. 4). David et al. [13] reported that the BI (%) of Cornelian cherry anthocyanins largely decreased during intestinal digestion, and the results were statistically significant (p < 0.05), while the antioxidant capacity of the extract was less affected by this phase of digestion.
Bioaccessibility index (BI) of fresh, SD, and FD CM samples SD solar dried, FD freeze-dried, TPC total phenolic contents, TFC total flavonoid contents, TAC total anthocyanin content, DPPH 2,2-diphenyl-1-picryhydrazyl radical scavenging activity, CUPRAC copper reducing antioxidant capacity, ABTS 2,2ʹ-azino-bis (3-ethyl benzothiazoline6-sulphonic acid), and FRAP ferric reducing antioxidant power. Means with different letters (a–c) among sample (fresh, SD, and FD fruits) are significantly different (p < 0.05) for TPC, TFC, TAC, ABTS, CUPRAC, DPPH, and FRAP
Changes in the levels of individual phenolics of fresh and dried Cornus mas during in vitro digestion
The effects of in vitro gastrointestinal digestion of CM phenolic compounds in the free and bound fractions were evaluated in the present study, and the results are summarized in Table 2. Generally, the amount of detected phenolics in the free fraction decreased from gastric to intestinal condition depending on the sample and was significantly different (p < 0.05) between the gastric (SGF) and intestinal (SIF) digestion steps for each sample. For example, the amount of epicatechin and catechin was decreased in all samples, whereas chlorogenic acid, gallic acid, rutin, and quercetin-3-D-galactoside were decreased in dried samples but not in fresh samples. Ellagic acid that was determined at higher levels in the extractable fraction of nondigested dried fruits could be detected in the intestinal fluids of SD fruit but not fresh or FD fruit.
The number and concentration of phenolics detected in the intestinal digestion fluids of fresh samples were higher than those of dried samples. For example, 3-hydroxybenzoic acid, epicatechin, quercetin-3-d-galactoside, rutin, and kaempferol 3-O-glucoside can be detected in fresh CM fruit, but in dried fruits, those phenolics could not be detected or determined at a comparably lower content. On the other hand, after gastrointestinal digestion, some phenolics, for example, ellagic acid, were detected at higher levels in SD fruit but not in fresh or FD fruit, and chlorogenic acid was determined at higher levels compared to those in the fresh sample.
The concentration of detected phenolics in the nondigested sample was also generally decreased from the gastric to the intestinal stage of digestion. For example, the content of gallic acid, epicatechin, rutin, catechin, ellagic acid, chlorogenic acid, and neochlorogenic acid were all decreased by digestion. They could be both released from the bound fraction and degraded during digestion simultaneously. The quercetin 3-D-galactoside (36.20–106.30%), chlorogenic acid (30.82–94.20%), and catechin (20.95–83.17%) showed the highest bioaccessibility for all samples (Table 2). The stability of phenolics in digestion conditions could be related to their structure. For example, the free flavonoid ( +)-catechin showed high bioaccessibility. This increase in the amount of flavonoid values by digestion might be related to the hydrolysis of the complex compounds (as allocated catechins) from their glycoside to aglycone forms [58]. Similar results were observed according to the literature [58, 73]. However, Ozkan et al. [28] and Chait et al. [58] reported the high bioaccessibility of chlorogenic acid in grapes and carob, respectively. Generally, the amount of detected anthocyanins in the free and bound fractions decreased from gastric to intestinal condition depending on the sample (Table 2). The cyanidin 3-O-glucoside and pelargonidin 3-O-glucoside were detected in the fresh and FD fruits at all digestion steps, while the cyanidin 3-O-rutinoside and peonidin 3-O-glucoside degraded at the gastric and intestinal steps. Although cyanidin 3-O-glucoside, pelargonidin 3-O-glucoside, and peonidin 3-O-glucoside were detected in SD fruit, cyanidin 3-O-rutinoside could not be determined at any digestion steps. Ozkan et al. [29] and Seke et al. [74] also reported a high decrease in anthocyanins after in vitro digestion. The BI (%) values of cyanidin 3-O-glucoside and pelargonidin 3-O-glucoside in the FD fruit were found to be 17.81% and 17.34%, respectively, and the BI % of FD was higher than other CM samples.
Conclusion
In this study, the FD was the preferred dehydration method in terms of the retention of phenolics, anthocyanins, and antioxidant capacities. Compared to fresh fruit, the proportion of bound phenolics in the total phenolic content and antioxidant capacities decreased with drying, possibly due to the weakened attachment of bound phenolics to the cell walls. When fresh and dried CM fruits were subjected to a simulated digestion model, the release of phenolics from the matrix and their degradation occurred simultaneously during digestion, and this could be influenced by the state of the fruit, whether fresh or dried. Future research could employ more comprehensive determination methods like LC–MS/MS to identify unknown constituents by providing structural and molecular weight characterization, given the complex structure of phenolic compounds and their potential transformation products during gastrointestinal digestion.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Dinda B, Kyriakopoulos AM, Dinda S, Zoumpourlis V, Thomaidis NS, Velegraki A, Markopoulos C, Dinda M (2016) Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2016.09.042
Bayram HM, Ozturkcan SA (2020) Bioactive components and biological properties of cornelian cherry (Cornus mas L.): a comprehensive review. J Funct Foods. https://doi.org/10.1016/j.jff.2020.104252
Hidalgo GI, Almajano MP (2017) Red fruits: extraction of antioxidants, phenolic content, and radical scavenging determination: a review. Antioxidants. https://doi.org/10.3390/antiox6010007
Bayram HM, Iliaz R, Gunes FE (2024) Effects of Cornus mas L. on anthropometric and biochemical parameters among metabolic associated fatty liver disease patients: randomized clinical trial. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2023.117068
Celik ZM, Sargin M, Tamer HG, Gunes FE (2023) The effect of lyophilized dried cornelian cherry (Cornus mas L.) intake on anthropometric and biochemical parameters in women with insulin resistance: a randomized controlled trial. Food Sci Nutr. https://doi.org/10.1002/fsn3.3725
Asgary S, Kelishadi R, Rafieian-Kopaei M, Najafi S, Najafi M, Sahebkar A (2013) Investigation of the lipid-modifying and antiinflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr Cardiol. https://doi.org/10.1007/s00246-013-0693-5
Gholamrezayi A, Aryaeian N, Rimaz S, Abolghasemi J, Fallah S, Moradi N, Taghizadeh M (2019) The effect of Cornus mas fruit extract consumption on lipid profile, glycemic indices, and leptin in postmenopausal women—a randomized clinical trial. Phytother Res. https://doi.org/10.1002/ptr.6476
Lietava J, Beerova N, Klymenko SV, Panghyova E, Varga I, Pechanova O (2019) Effects of cornelian cherry on atherosclerosis and its risk factors. Oxid Med Cell Longev. https://doi.org/10.1155/2019/2515270
Petkova NT, Ognyanov MH (2018) Phytochemical characteristics and in vitro antioxidant activity of fresh, dried and processed fruits of Cornelian cherries (Cornus mas L.). Bulg Chem Commun 50:302–307
Nunes JC, Lago MG, Castelo-Branco VN, Oliveira FR, Guedes Torres A, Perrone D, Monteiro M (2016) Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem. https://doi.org/10.1016/j.foodchem.2015.11.050
Aydin E, Gocmen D (2015) The influences of drying method and metabisulfite pre-treatment on the color, functional properties and phenolic acids contents and bioaccessibility of pumpkin flour. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2014.08.025
Silva-Espinoza MA, Ayed C, Foster T, del Mar CM, Martínez-Navarrete N (2020) The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree. Foods. https://doi.org/10.3390/foods9010032
David L, Danciu V, Moldovan B, Filip A (2019) Effects of in vitro gastrointestinal digestion on the antioxidant capacity and anthocyanin content of cornelian cherry fruit extract. Antioxidants. https://doi.org/10.3390/antiox8050114
Radbeh Z, Asefi N, Hamishehkar H, Roufegarinejad L, Pezeshki A (2020) Novel carriers ensuring enhanced anti-cancer activity of Cornus mas (Cornelian cherry) bioactive compounds. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2020.109906
Cemeroglu B (1992) Meyve ve sebze işleme endüstrisinde temel analiz metotları. Ankara, Turkey
da Silva Haas IC, Toaldo IM, Gomes TM, Luna AS, de Gois JS, Bordignon-Luiz MT (2019) Polyphenolic profile, macro-and microelements in bioaccessible fractions of grape juice sediment using in vitro gastrointestinal simulation. Food Biosci. https://doi.org/10.1016/j.fbio.2018.11.002
Arruda HS, Pereira GA, de Morais DR, Eberlin MN, Pastore GM (2018) Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. https://doi.org/10.1016/j.foodchem.2017.11.120
Yu J, Li W, You B, Yang S, **an W, Deng Y, Huang W, Yang R (2021) Phenolic profiles, bioaccessibility and antioxidant activity of plum (Prunus salicina Lindl). Food Res Int. https://doi.org/10.1016/j.foodres.2021.110300
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. https://doi.org/10.1016/S0076-6879(99)99017-1
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. https://doi.org/10.1016/S0308-8146(98)00102-2
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. CPFAC. https://doi.org/10.1002/0471142913.faf0102s00
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. https://doi.org/10.1016/S0023-6438(95)80008-5
Apak R, Güçlü K, Özyürek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. https://doi.org/10.1021/jf048741x
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. https://doi.org/10.1016/S0891-5849(98)00315-3
Benzie IFF, Strain JJ (1999) The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal Biochem. https://doi.org/10.1006/abio.1996.0292
Ozcan BE, Saroglu O, Karakas CY, Karadag A (2023) Encapsulation of purple basil leaf extract by electrospraying in double emulsion (w/o/w) filled alginate-carrageenan beads to improve the bioaccessibility of anthocyanins. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2023.126207
Ozkan K, Karadag A, Sagdic O, Ozcan FS, Ozer H (2023) The effects of different drying methods on the sugar, organic acid, volatile composition, and textural properties of black ‘Isabel’grape. J Food Meas Charact. https://doi.org/10.1007/s11694-022-01740-4
Brodkorb A, Egger L, Alminger M, Alvito P, Assunçao R, Ballance S, Bohn T, Bourlieu-Lacanal C, Boutrou R, Carriere F, Clemente A, Corredig M, Dupont D, Edwards C, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie AR, Martins C, Marze S, McClements DJ, Menard O, Minekus M, Portmann R, Santos CN, Souchon I, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W, Recio I (2019) INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc. https://doi.org/10.1038/s41596-018-0119-1
Ozkan K, Karadag A, Sagdic O (2022) The effects of different drying methods on the in vitro bioaccessibility of phenolics, antioxidant capacity, minerals and morphology of black ‘Isabel’grape. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2022.11318
Miranda M, Vega-Gálvez A, López J, Parada G, Sanders M, Aranda M, Uribe E, Di Scala K (2010) Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.). Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2010.04.019
Kulapichitr F, Borompichaichartkul C, Suppavorasatit I, Cadwallader KR (2019) Impact of drying process on chemical composition and key aroma components of Arabica coffee. Food Chem. https://doi.org/10.1016/j.foodchem.2019.03.152
Tural S, Koca I (2008) Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci Hortic. https://doi.org/10.1016/j.scienta.2008.02.003
Ozrenk K, Tas A, Gundogdu M, Keskin N, Ercisli S (2023) physicochemical substances and bioactive components of wild cornelian cherry (Cornus mas L.) fruits in Erzincan province of Eastern Turkey. Genetika. https://doi.org/10.2298/GENSR2301095K
Rosu CM, Olteanu Z, Truta E, Ciornea E, Manzu C, Zamfirache MM (2011) Nutritional value of Rosa spp. L. and Cornus mas L. fruits, as affected by storage conditions. J Exp Molec Biol 14:147–155
Güleryüz M, Bolat I, Pırlak L (1998) Selection of table cornelian cherry (Cornus mas L.) types in Coruh Valley. Turk J Agric For 22:357–364
Demir F, Kalyoncu IH (2003) Some nutritional, pomological and physical properties of cornelian cherry (Cornus mas L.). J Food Eng. https://doi.org/10.1016/S0260-8774(03)00056-6
Pırlak L, Guleryuz M, Bolat I (2003) Promising Cornelian Cherries From the Northeastern Anatolia 57:14–18
Aslantas R, Pirlak L, Güleryüz M (2007) The nutritional value of wild fruits from the North Eastern Anatolia region of Turkey. Asian J Chem 19:3072–2078
Nowak D, Jakubczyk E (2020) The freeze-drying of foods—the characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 9(10):1488. https://doi.org/10.3390/foods9101488
Szczepaniak O, Cielecka-Piontek J, Kobus-Cisowska J (2021) Hypoglycaemic, antioxidative and phytochemical evaluation of Cornus mas varieties. Eur Food Res Technol. https://doi.org/10.1007/s00217-020-03616-7
Gozlekci S, Esringu A, Ercisli S, Eyduran SP, Akin M, Bozovic D, Sagbas HI (2017) Mineral content of cornelian cherry (Cornus mas L.) fruits. Oxid Commun 40:301–308
Ochmian I, Oszmiański J, Lachowicz S, Krupa-Małkiewicz M (2019) Rootstock effect on physico-chemical properties and content of bioactive compounds of four cultivars Cornelian cherry fruits. Sci Hortic. https://doi.org/10.1016/j.scienta.2019.108588
Mingroni TT, Hamada J, Xavier AD, Cavalcante C, Nascimento AND (2019) In vitro evaluation of Ca, Cu, and Mg bioaccessibility in fresh and dried fruits. J Braz Chem Soc. https://doi.org/10.21577/0103-5053.20180159
Sun Y, Shen Y, Liu D, Ye X (2015) Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2014.09.001
Garcìa LM, Ceccanti C, Negro C, De Bellis L, Incrocci L, Pardossi A, Guidi L (2021) Effect of drying methods on phenolic compounds and antioxidant activity of Urtica dioica L. leaves. Hortic. https://doi.org/10.3390/horticulturae7010010
Ucar TM, Karadag A (2019) The effects of vacuum and freeze-drying on the physicochemical properties and in vitro digestibility of phenolics in oyster mushroom (Pleurotus ostreatus). J Food Meas Charact. https://doi.org/10.1007/s11694-019-00149-w
Giambanelli E, Gómez-Caravaca AM, Ruiz-Torralba A, Guerra-Hernández EJ, Figueroa-Hurtado JG, García-Villanova B, Verardo V (2020) New advances in the determination of free and bound phenolic compounds of Banana Passion Fruit Pulp (Passiflora tripartita, var. Mollissima (Kunth) LH Bailey) and their in vitro antioxidant and hypoglycemic capacities. Antioxidants. https://doi.org/10.3390/antiox9070628
Li M, Chen X, Deng J, Ouyang D, Wang D, Liang Y, Chen Y, Sun Y (2020) Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127429
Dibanda RF, Akdowa EP, Tongwa QM (2020) Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. https://doi.org/10.1016/j.foodchem.2019.125308
Hu X, Yang T, Qi X, Guo X, Hu J (2022) Effects of different drying methods on phenolic composition and antioxidant activity in corn silk (Stigma maydis). J Food Process Preserv. https://doi.org/10.1111/jfpp.17101
Martinović A, Cavoski I (2020) The exploitation of cornelian cherry (Cornus mas L.) cultivars and genotypes from Montenegro as a source of natural bioactive compounds. Food Chem. https://doi.org/10.1016/j.foodchem.2020.126549
Deng S, West BJ, Jensen CJ (2013) UPLC-TOF-MS characterization and identification of bioactive iridoids in Cornus mas fruit. J Anal Methods Chem. https://doi.org/10.1155/2013/710972
Natić M, Pavlović A, Bosco FL, Stanisavljević N, Zagorac DD, Akšić MF, Papetti A (2019) Nutraceutical properties and phytochemical characterization of wild Serbian fruits. Eur Food Res Technol. https://doi.org/10.1007/s00217-018-3178-1
Drkenda P, Spahic A, Begic-Akagic A, Gasi F, Vranac A, Blanke M (2014) Pomological characteristics of some autochthonous genotypes of cornelian cherry (Cornus mas L.) in Bosnia and Herzegovina. Erwerbs-obstbau. https://doi.org/10.1007/s10341-014-0203-9
Chen PX, Tang Y, Marcone MF, Pauls PK, Zhang B, Liu R, Tsao R (2015) Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular-and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. https://doi.org/10.1016/j.foodchem.2015.03.100
You B, Yang S, Yu J, **an W, Deng Y, Huang W, Li W, Yang R (2021) Effect of thermal and dry salt-curing processing on free and bound phenolics and antioxidant activity in Prunus mume fruits together with the phenolic bioaccessibility. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2021.111355
Yao J, Chen J, Yang J, Hao Y, Fan Y, Wang C, Li N (2021) Free, soluble-bound and insoluble-bound phenolics and their bioactivity in raspberry pomace. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2020.109995
Chait YA, Gunenc A, Bendali F, Hosseinian F (2020) Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: bioaccessibility and bioactivity. LWT-Food Sci Technol. https://doi.org/10.1016/j.lwt.2019.108623
Seke F, Manhivi VE, Shoko T, Slabbert RM, Sultanbawa Y, Sivakumar D (2021) Effect of freeze drying and simulated gastrointestinal digestion on phenolic metabolites and antioxidant property of the Natal plum (Carissa macrocarpa). Foods. https://doi.org/10.3390/foods10061420
Wootton-Beard PC, Moran A, Ryan L (2011) Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int. https://doi.org/10.1016/j.foodres.2010.10.033
Baker I, Chohan M, Opara EI (2013) Impact of cooking and digestion, in vitro, on the antioxidant capacity and anti-inflammatory activity of cinnamon, clove and nutmeg. Plant Foods Hum Nutr. https://doi.org/10.1007/s11130-013-0379-4
Martínez-Las Heras R, Pinazo A, Heredia A, Andrés A (2017) Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. https://doi.org/10.1016/j.foodchem.2016.07.104
Zhang B, Deng Z, Tang Y, Chen PX, Liu R, Ramdath DD, Liu Q, Hernandez M, Tsao R (2017) Bioaccessibility, in vitro antioxidant and anti-inflammatory activities of phenolics in cooked green lentil (Lens culinaris). J Funct Foods. https://doi.org/10.1016/j.jff.2017.03.004
Górnaś P, Dwiecki K, Siger A, Tomaszewska-Gras J, Michalak M, Polewski K (2016) Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur Food Res Technol. https://doi.org/10.1007/s00217-015-2572-1
Miletić N, Mitrović O, Popović B, Nedović V, Zlatković B, Kandić M (2013) Polyphenolic content and antioxidant capacity in fruits of plum (Prunus domestica L.) cultivars “Valjevka” and “Mildora” as influenced by air drying. J Food Qual. https://doi.org/10.1111/jfq.12035
Adom KK, Liu RH (2002) Antioxidant activity of grains. J Agric Food Chem. https://doi.org/10.1021/jf0205099
Pérez-Jiménez J, Díaz-Rubio ME, Saura-Calixto F (2013) Non-extractable polyphenols, a major dietary antioxidant: occurrence, metabolic fate and health effects. Nutr Res Rev. https://doi.org/10.1017/S0954422413000097
Lee HY, Jang Y, Koh E (2022) Effect of drying methods on in vitro digestion stability of anthocyanins and polyphenols from Omija (schisandra Chinensis Baillon). J Food Process Preserv. https://doi.org/10.1111/jfpp.17055
Gullon B, Pintado ME, Barber X, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M (2015) Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res Int. https://doi.org/10.1016/j.foodres.2015.10.021
Burgos-Edwards A, Jiménez-Aspee F, Thomas-Valdés S, Schmeda-Hirschmann G, Theoduloz C (2017) Qualitative and quantitative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastrointestinal digestion. Food Chem. https://doi.org/10.1016/j.foodchem.2017.06.060
Odriozola-Serrano I, Nogueira DP, Esparza I, Vaz AA, Jiménez-Moreno N, Martín-Belloso O, Ancín-Azpilicueta C (2023) Stability and bioaccessibility of phenolic compounds in rosehip extracts during in vitro digestion. Antioxidants. https://doi.org/10.3390/antiox12051035
Bermúdez-Soto MJ, Tomás-Barberán FA, García-Conesa MT (2007) Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. https://doi.org/10.1016/j.foodchem.2006.06.025
Celep E, İnan Y, Akyüz S, Yesilada E (2017) The bioaccessible phenolic profile and antioxidant potential of Hypericum perfoliatum L. after simulated human digestion. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2017.09.032
Seke F, Manhivi VE, Shoko T, Slabbert RM, Sultanbawa Y, Sivakumar D (2021) Extraction optimisation, hydrolysis, antioxidant properties and bioaccessibility of phenolic compounds in Natal plum fruit (Carissa macrocarpa). Food Biosci. https://doi.org/10.1016/j.fbio.2021.101425
Acknowledgements
This work has been financially supported by the Istanbul Gelisim University Scientific Research Projects (BAP) under the Project Number of KAP-150121-SAO.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not present studies involving human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bayram, H.M., Ozkan, K., Ozturkcan, A. et al. Effect of drying methods on free and bound phenolic compounds, antioxidant capacities, and bioaccessibility of Cornelian cherry. Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04552-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00217-024-04552-6