Abstract
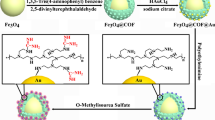
In this work, on the basis of an immobilized metal ion affinity chromatography enrichment strategy, a new kind of covalent organic framework (COF) material for enrichment of phosphorylated peptides and exosomes was successfully prepared in a facile method, and Ti4+ and Nb5+ were used as dual-functional ions (denoted as COF-S–S-COOH-Ti4+/Nb5+). With the advantage of unbiased enrichment towards phosphopeptides, COF-S–S-COOH-Ti4+/Nb5+ shows ultra-high selectivity (maximum molar ratio of β-casein: BSA is 1:20,000) and low limit of detection (0.2 fmol). In addition, the material has an excellent phosphopeptide loading capacity (100 μg/mg) and reusability (at least seven times). Furthermore, applying the material to the actual sample, 4 phosphopeptides were selectively extracted from the serum of renal carcinoma patients. At the same time, exosomes with an intact structure in the serum of renal carcinoma patients were successfully isolated rapidly using this strategy. All experiments have shown that COF-S–S-COOH-Ti4+/Nb5+ exhibits exciting potential in practical applications.
Graphical abstract









Similar content being viewed by others
References
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. https://doi.org/10.1126/science.aau6977.
Zhang N, Sun NR, Deng CH. A hydrophilic magnetic MOF for the consecutive enrichment of exosomes and exosomal phosphopeptides. Chem Commun. 2020;56(90):13999–4002. https://doi.org/10.1039/d0cc06147f.
Zhang LN, Gu CC, Wen JJ, Liu GX, Liu HY, Li LH. Recent advances in nanomaterial-based biosensors for the detection of exosomes. Anal Bioanal Chem. 2021;413:83–102. https://doi.org/10.1007/s00216-020-03000-0.
Vella LJ, Scicluna BJ, Cheng L, Bawden EG, Masters CL, Ang CS, Willamson N, McLean C, Barnham KJ, Hill AF. A rigorous method to enrich for exosomes from brain tissue. J Extracell Vesicles. 2017;6(1). https://doi.org/10.1080/20013078.2017.1348885.
Zhang N, Hu XF, Chen HL, Deng CH, Sun NAR. Specific enrichment and glycosylation discrepancy profiling of cellular exosomes using a dual-affinity probe. Chem Commun. 2021;57(51):6249–52. https://doi.org/10.1039/d1cc01530c.
Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci. 2016;17(2):171. https://doi.org/10.3390/ijms17020171.
Zhao XX, Zhang WQ, Qiu XP, Mei Q, Luo Y, Fu WL. Rapid and sensitive exosome detection with CRISPR/Cas12a. Anal Bioanal Chem. 2020;412:601–9. https://doi.org/10.1007/s00216-019-02211-4.
Zhang N, Sun NR, Deng CH. Rapid isolation and proteome analysis of urinary exosome based on double interactions of Fe3O4@TiO2-DNA aptamer. Talanta. 2021;221:121571. https://doi.org/10.1016/j.talanta.2020.121571.
Wu YL, Liu QJ, Deng CH. L-cysteine-modified metal-organic frameworks as multifunctional probes for efficient identification of N-linked glycopeptides and phosphopeptides in human crystalline lens. Anal Chim Acta. 2019;1061:110–21. https://doi.org/10.1016/j.aca.2019.01.052.
Jiang JB, Sun XN, She XJ, Li JJ, Li Y, Deng CH, Duan GL. Magnetic microspheres modified with Ti(IV) and Nb(V) for enrichment of phosphopeptides. Microchim Acta. 2018;185:309–16. https://doi.org/10.1007/s00604-018-2837-z.
Chen C, Zhang XF, Dong XF, Zhou H, Li XL, Liang XM. TiO2 simultaneous enrichment, on-line deglycosylation, and sequential analysis of Glyco- and phosphopeptides. Front Chem. 2019;9. https://doi.org/10.3389/fchem.2021.703176.
Wang ZD, Wang JW, Sun NR, Deng CH. A promising nanoprobe based on hydrophilic interaction liquid chromatography and immobilized metal affinity chromatography for capture of glycopeptides and phosphopeptides. Anal Chim Acta. 2019;1067:1–10. https://doi.org/10.1016/j.aca.2019.04.010.
Sun NR, Wang ZD, Wang JW, Chen HM, Wu H, Shen S, Deng CH. Hydrophilic tripeptide combined with magnetic titania as a multipurpose platform for universal enrichment of phospho- and glycopeptides. J Chromatogr A. 2019;1595:1–10. https://doi.org/10.1016/j.chroma.2019.02.039.
Wang BC, Yan YH, Ding CF. Metal organic frameworks as advanced adsorbent materials for separation and analysis of complex samples. J Chromatogr A. 2022;1671: 462971. https://doi.org/10.1016/j.chroma.2022.462971.
Li YL, Liu LL, Wu H, Deng CH. Magnetic mesoporous silica nanocomposites with binary metal oxides core-shell structure for the selective enrichment of endogenous phosphopeptides from human saliva. Anal Chim Acta. 2019;1079:111–9. https://doi.org/10.1016/j.aca.2019.06.045.
Wu YL, Liu QJ, **e YQ, Deng CH. Core-shell structured magnetic metal-organic framework composites for highly selective enrichment of endogenous N-linked glycopeptides and phosphopeptides. Talanta. 2018;190:298–312. https://doi.org/10.1016/j.talanta.2018.08.010.
Grassetti AV, Hards R, Gerber SA. Offline pentafluorophenyl (PFP)-RP prefractionation as an alternative to high-pH RP for comprehensive LC-MS/MS proteomics and phosphoproteomics. Anal Bioanal Chem. 2017;409:4615–25. https://doi.org/10.1007/s00216-017-0407-6.
Bijttebier S, Theunis C, Jahouh F, Martins DR, Verhemeldonck M, Grauwen K, Dillen L, Mercken M. Development of immunoprecipitation-two-dimensional liquid chromatography-mass spectrometry methodology as biomarker read-out to quantify phosphorylated tau in cerebrospinal fluid from Alzheimer disease patients. J Chromatogr A. 2021;1651: 462299. https://doi.org/10.1016/j.chroma.2021.462299.
Hennrich ML, Groenewold V, Kops GJ, Heck AJ, Mohammed S. Improving depth in phosphoproteomics by using a strong cation exchange-weak anion exchange-reversed phase multidimensional separation approach. Anal Chem. 2011;83:7137–43. https://doi.org/10.1021/ac2015068.
**ao RL, Pan YN, Li J, Zhang LY, Zhang WB. Layer-by-layer assembled magnetic bimetallic metal-organic framework composite for global phosphopeptide enrichment. J Chromatogr A. 2019;1601:45–52. https://doi.org/10.1016/j.chroma.2019.05.010.
Liu XY, Rossio V, Thakurta SG, Flora A, Foster L, Bomgarden RD, Gygi SP, Paulo JA. Fe3+-NTA magnetic beads as an alternative to spin column-based phosphopeptide enrichment. J Proteomics. 2022;260:104561. https://doi.org/10.1016/j.jprot.2022.104561.
Zhu CH, Wu JN, ** XT, Yan YH, Ding CF, Tang KQ, Zhang D. Post-synthesis of biomimetic chitosan with honeycomb-like structure for sensitive recognition of phosphorylated peptides. J Chromatogr A. 2021;1643: 462072. https://doi.org/10.1016/j.chroma.2021.462072.
Zhang KN, Hu DH, Deng SM, Han M, Wang XF, Liu HL, Liu Y, **e MX. Phytic acid functionalized Fe3O4 nanoparticles loaded with Ti(IV) ions for phosphopeptide enrichment in mass spectrometric analysis. Microchim Acta. 2019;186(2):1. https://doi.org/10.1007/s00604-018-3177-8.
Jiang DD, Li Z, Jia QA. Sensitive and selective phosphopeptide enrichment strategy by combining polyoxometalates and cysteamine hydrochloride-modified chitosan through layer-by-layer assembly. Anal Chim Acta. 2019;1066:58–68. https://doi.org/10.1016/j.aca.2019.04.001.
Yan S, Luo B, He J, Lan F, Wu Y. Phytic acid functionalized magnetic bimetallic metal-organic frameworks for phosphopeptide enrichment. J Mater Chem B. 2021;9(7):1811–20. https://doi.org/10.1039/d0tb02517h.
Cho KY, Chen LJ, Hu YW, Schnaubelt M, Zhang H. Develo** workflow for simultaneous analyses of phosphopeptides and glycopeptides. ACS Chem Biol. 2019;14(1):58–66. https://doi.org/10.1021/acschembio.8b00902.
Bae SW, Kim JI, Choi I, Sung J, Hong JI, Yeo WS. Zinc ion-immobilized magnetic microspheres for enrichment and identification of multi-phosphorylated peptides by mass spectrometry. Anal Sci. 2017;33:1381–6. https://doi.org/10.2116/analsci.33.1381.
Gao CH, Bai J, He YT, Zheng Q, Ma WD, Lei ZX, Zhang MY, Wu J, Fu FF, Lin Z. Postsynthetic functionalization of Zr4+-immobilized core-shell structured magnetic covalent organic frameworks for selective enrichment of phosphopeptides. ACS Appl Mater Interfaces. 2019;11(14):13735–41. https://doi.org/10.1021/acsami.9b03330.
Wahid A, Sohail A, Wang HY, Guo M, Zhang L, Ji Y, Wang P, **ao H. Titanium(IV) immobilized affinity chromatography facilitated phosphoproteomics analysis of salivary extracellular vesicles for lung cancer. Anal Bioanal Chem. 2022;414(12):3697–708. https://doi.org/10.1007/s00216-022-04013-7.
Posewitz MC, Tempst P. Immobilized gallium (III) affinity chromatography of phosphopeptides. Anal Chem. 1999;71(14):2883–92. https://doi.org/10.1021/ac981409y.
Gao R, Li J, Shi R, Zhang Y, Ouyang FZ, Zhang T, Hu LH, Xu GQ, Liu J. Highly sensitive detection of phosphopeptides with superparamagnetic Fe3O4@mZrO(2) core-shell microspheres-assisted mass spectrometry. J Mater Sci Technol. 2020;59:234–42. https://doi.org/10.1016/j.jmst.2020.02.091.
Lin HZ, Chen HM, Shao X, Deng CH. A capillary column packed with a zirconium (IV)-based organic framework for enrichment of endogenous phosphopeptides. Microchim Acta. 2018;185(12):562. https://doi.org/10.1007/s00604-018-3109-7.
Lin HZ, Deng CH. Development of Hf4+-immobilized polydopamine-coated magnetic graphene for highly selective enrichment of phosphopeptides. Talanta. 2016;149:91–7. https://doi.org/10.1016/j.talanta.2015.11.037.
Dai JY, Wang MD, Liu HL. Highly selective enrichment of phosphopeptides using Zr4+-immobilized Titania nanoparticles. Talanta. 2017;164:222–7. https://doi.org/10.1016/j.talanta.2016.11.058.
Liu QJ, Sun NR, Gao MX, Deng CH. Magnetic binary metal–organic framework as a novel affinity probe for highly selective capture of endogenous phosphopeptides. ACS Sustain Chem Eng. 2018;6:4382–9. https://doi.org/10.1021/acssuschemeng.8b00023.
Zheng HY, Wang JX, Gao MX, Zhang XM. Titanium (IV)-functionalized zirconium-organic frameworks as dual-metal affinity probe for recognition of endogenous phosphopeptides prior to mass spectrometric quantification. Microchim Acta. 2019;186(12):829–39. https://doi.org/10.1007/s00604-019-3962-z.
Liu B, Wang BC, Yan YH, Tang KQ, Ding CF. Efficient separation of phosphopeptides employing a Ti/Nb-functionalized core-shell structure solid-phase extraction nanosphere. Microchim Acta. 2021;188(2):32. https://doi.org/10.1007/s00604-020-04652-6.
Zhai R, Tian F, Xue RQ, Jiao FL, Hao FR, Zhang YJ, Qian XH. Metal ion-immobilized magnetic nanoparticles for global enrichment and identification of phosphopeptides by mass spectrometry. RSC Adv. 2016;6:1670–7. https://doi.org/10.1039/c5ra22006h.
Cheng JM, Hu K, Liu QR, Liu YJ, Yang HX, Kong JM. Electrochemical ultrasensitive detection of CYFRA21-1 using Ti3C2Tx-MXene as enhancer and covalent organic frameworks as labels. Anal Bioanal Chem. 2021;413:2543–51. https://doi.org/10.1007/s00216-021-03212-y.
Wang HP, Jiao FL, Gao FY, Lv YY, Wu Q, Zhao Y, Shen YH, Zhang YJ, Qian XH. Titanium (IV) ion-modified covalent organic frameworks for specific enrichment of phosphopeptides. Talanta. 2017;166:133–40. https://doi.org/10.1016/j.talanta.2017.01.043.
Ma WD, Zheng Q, He YT, Li GR, Guo WJ, Lin Z, Zhang L. Size-controllable synthesis of uniform spherical covalent organic frameworks at room temperature for highly efficient and selective enrichment of hydrophobic peptides. J Am Chem Soc. 2019;141(45):18271–7. https://doi.org/10.1021/jacs.9b09189.
Sun Q, Aguila B, Perman J, Earl LD, Abney CW, Cheng YC, Wei H, Nguyen N, Wojtas L, Ma SQ. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J Am Chem Soc. 2017;139(7):2786–93. https://doi.org/10.1021/jacs.6b12885.
Funding
This work is supported by the National Natural Science Foundation of China (21927805), Natural Science Foundation of Zhejiang Province (LY22B050008), Major science and technology projects in Ningbo (2020Z090), and the K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
Ethically approved human serums used in this research were collected with the consent of volunteers. The study was conducted with the approval of the experimental ethics committee of Ningbo University and its affiliated hospital.
Consent for publication
All of the authors have given approval for the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Feng, Q., **e, Z. et al. A Ti/Nb-functionalized COF material based on IMAC strategy for efficient separation of phosphopeptides and phosphorylated exosomes. Anal Bioanal Chem 414, 7885–7895 (2022). https://doi.org/10.1007/s00216-022-04323-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04323-w