Abstract
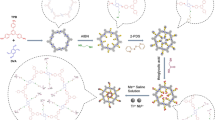
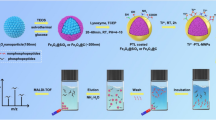
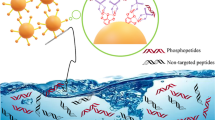
A guanidine-functionalized (GF) covalent organic framework (COF) nanocomposite has been developed by a post-synthetic approach for specific capture and separation of phosphopeptides and exosomes. The abundant binding sites on COF can immobilize a large number of gold nanoparticles (AuNPs), which can be used to react with amino groups to graft polyethyleneimine (PEI). Finally, Fe3O4@COF@Au@PEI-GF is obtained through the reaction of PEI and guanidyl group for phosphopeptides and exosomes detection. This composite shows a low detection limit (0.02 fmol), size exclusion effect (β-casein digests:Albumin from bovine serum protein = 1:10,000), good reusability (10 cycles), and high selectivity (β-casein digests:Albumin from bovine serum digests = 1:10,000). For complex biological sample, 4 phosphopeptides can be successfully identified from human serum. Furthermore, for the first time, we used guanidyl-functionalized probe to capture exosomes in human serum, providing a new method for enriching exosomes. The above experiments showed that Fe3O4@COF@Au@PEI-GF not only effectively enrich phosphopeptides and remove macromolecular proteins, but also successfully separate and capture exosomes. This demonstrates the great potential of this composite for the specific enrichment of phosphopeptides and isolation of exosomes.
Graphical abstract







Similar content being viewed by others
References
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Worms DP, Kalluri R (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523:177-U82. https://doi.org/10.1038/nature14581
Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ (2012) Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56:293–304. https://doi.org/10.1016/j.ymeth.2012.01.002
Wei HX, Chen JY, Wang SL, Fu FH, Zhu X, Wu CY, Liu ZJ, Zhong GX, Lin JH (2019) A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int J Nanomedicine 14:8603–8610. https://doi.org/10.2147/IJN.S218988
Webber J, Steadman R, Mason MD, Tabi Z, Clayton A (2010) Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 70:9621–9630. https://doi.org/10.1158/0008-5472.CAN-10-1722
**ong FF, Jia JX, Ma JT, Jia Q (2022) Glutathione-functionalized magnetic thioether-COFs for the simultaneous capture of urinary exosomes and enrichment of exosomal glycosylated and phosphorylated peptides. Nanoscale 14:853–864. https://doi.org/10.1039/d1nr06587d
Kang YT, Kim YJ, Bu J, Cho YH, Han SW, Moon BI (2017) High-purity capture and release of circulating exosomes using an exosome-specific dual-patterned immunofiltration (ExoDIF) device. Nanoscale 9:13495–13505. https://doi.org/10.1039/c7nr04557c
Hwang DW (2019) Perspective in nuclear theranostics using exosome for the brain. Med Mol Imaging 53:108–114. https://doi.org/10.1007/s13139-018-00567-6
Lin SJ, Yu ZX, Chen D, Wang ZG, Miao JM, Li QC, Zhang DY, Song J, Cui DX (2020) Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 16:1903916. https://doi.org/10.1002/smll.201903916
Singh K, Nalabotala R, Koo KM, Bose S, Nayak R, Shiddiky MJA (2021) Separation of distinct exosome subpopulations: isolation and characterization approaches and their associated challenges. Analyst 146:3731–3749. https://doi.org/10.1039/d1an00024a
Sun NR, Yu HL, Wu H, Shen XZ, Deng CH (2021) Advanced nanomaterials as sample technique for bio-analysis. Trac-Trends Anal Chem 135:116168. https://doi.org/10.1016/j.trac.2020.116168
Khodashenas S, Khalili S, Moghadam MF (2019) A cell ELISA based method for exosome detection in diagnostic and therapeutic applications. Biotechnol Lett 41:523–531. https://doi.org/10.1007/s10529-019-02667-5
Riva P, Battaglia C, Venturin M (2019) Emerging role of genetic alterations affecting exosome biology in neurodegenerative diseases. Int J Mol Sci 20:4113. https://doi.org/10.3390/ijms20174113
Zhang N, Hu XF, Chen HL, Deng CH, Sun NAR (2021) Specific enrichment and glycosylation discrepancy profiling of cellular exosomes by dual-affinity probe. Chem Commun 57:6249–6252. https://doi.org/10.1039/d1cc01530c
Zhang N, Sun NR, Deng CH (2020) A hydrophilic magnetic MOF for the consecutive enrichment of exosomes and exosomal phosphopeptides. Chem Commun 56:13999–14002. https://doi.org/10.1039/d0cc06147f
Cao F, Gao Y, Chu Q, Wu Q, Zhao L, Lan T, Zhao L (2019) Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis 40:3092–3098. https://doi.org/10.1002/elps.201900295
Sun NR, Wang JW, Yao Z, Chen HM, Deng CH (2019) Magnetite nanoparticles coated with mercaptosuccinic acid-modified mesoporous titania as a hydrophilic sorbent for glycopeptides and phosphopeptides prior to their quantitation by LC-MS/MS. Microchim Acta 186:159. https://doi.org/10.1007/s00604-019-3274-3
Sun NR, Wang ZD, Wang JW, Chen HM, Wu H, Shen S, Deng CH (2019) Hydrophilic tripeptide combined with magnetic titania as a multipurpose platform for universal enrichment of phospho- and glycopeptides. J Chromatogr A 1595:1–10. https://doi.org/10.1016/j.chroma.2019.02.039
Liu B, Wang BC, Yan YH, Tang KQ, Ding CF (2021) Efficient separation of phosphopeptides employing a Ti/Nb-functionalized core-shell structure solid-phase extraction nanosphere. Microchim Acta 188:32. https://doi.org/10.1007/s00604-020-04652-6
Chu HM, Zheng HY, Yao JZ, Sun NR, Yan GQ, Deng CH (2020) Magnetic metal phenolic networks: expanding the application of a promising nanoprobe to phosphoproteomics research. Chem Commun 56:11299–11302. https://doi.org/10.1039/d0cc04615a
Du JL, Yan YH, Tang KQ, Ding CF (2021) Modified carbon nanotubes decorated with ZIFs as new immobilized metal ion affinity chromatography platform for enrichment of phosphopeptides. ChemistrySelect 6:1313–1319. https://doi.org/10.1002/slct.202004650
Luo B, Zhou XX, Jiang PP, Yi QY, Lan F, Wu Y (2018) PAMA-Arg brushes-functionalized magnetic composite nanospheres for highly effective enrichment of the phosphorylated biomolecules. J Mater Chem B 6:3969–3978. https://doi.org/10.1039/c8tb00705e
Jiang DD, Duan LM, Jia Q, Liu JH (2020) Glycocyamine functionalized magnetic layered double hydroxides with multiple affinity sites for trace phosphopeptides enrichment. Anal Chim Acta 1136:25–33. https://doi.org/10.1016/j.aca.2020.07.057
**e ZH, Yan YH, Tang KQ, Ding CF (2022) Post-synthesis modification of covalent organic frameworks for ultrahigh enrichment of low-abundance glycopeptides from human saliva and serum. Talanta 236:122831. https://doi.org/10.1016/j.talanta.2021.122831
Gao CH, Bai J, He YT, Zheng Q, Ma WD, Lei ZX, Zhang MY, Wu J, Fu FF, Lin Z (2019) Postsynthetic functionalization of Zr4+-immobilized core−shell structured magnetic covalent organic frameworks for selective enrichment of phosphopeptides. ACS Appl Mater Interfaces 11:13735–13741. https://doi.org/10.1021/acsami.9b03330
Chen L, He YT, Lei ZX, Gao CL, **e Q, Tong P, Lin Z (2018) Preparation of core-shell structured magnetic covalent organic framework nanocomposites for magnetic solid-phase extraction of bisphenols from human serum sample. Talanta 181:296–304. https://doi.org/10.1016/j.talanta.2018.01.036
You LJ, Xu K, Ding GJ, Shi XM, Li JM, Wang SY, Wang JB (2020) Facile synthesis of Fe3O4@COF covalent organic frameworks for the adsorption of bisphenols from aqueous solution. J Mol Liq 320:114456. https://doi.org/10.1016/j.molliq.2020.114456
Baldwin LA, Crowe JW, Pyles DA, McGrier PL (2016) Metalation of a mesoporous three-dimensional covalent organic framework. J Am Chem Soc 138:15134–15137. https://doi.org/10.1021/jacs.6b10316
Lu YY, Wang XL, Wang LL, Zhang W, Wei JJ, Lin JM, Zhao RS (2021) Room-temperature synthesis of amino-functionalized magnetic covalent organic frameworks for efficient extraction of perfluoroalkyl acids in environmental water samples. J Hazard Mater 407:124782. https://doi.org/10.1016/j.jhazmat.2020.124782
Wang JX, Li J, Gao MX, Zhang XM (2018) Recent advances in covalent organic frameworks for separation and analysis of complex samples. TrAC Trends Anal Chem 108:98–109. https://doi.org/10.1016/j.trac.2018.07.013
Fu QB, Jiang HL, Qiao LQ, Sun X, Wang ML, Zhao RS (2020) Effective enrichment and detection of trace polybrominated diphenyl ethers in water samples based on magnetic covalent organic framework nanospheres coupled with chromatography-mass spectrometry. J Chromatogr A 1630:461534. https://doi.org/10.1016/j.chroma.2020.461534
Ren JF, Shen S, Pang ZQ, Lu XH, Deng CH, Jiang XG (2011) Facile synthesis of superparamagnetic Fe3O4@Au nanoparticles for photothermal destruction of cancer cells. Chem Commun 47:11692–11694. https://doi.org/10.1039/c1cc15528h
Ma YY, Zhao YX, Xu XT, Ding SJ, Li YH (2021) Magnetic covalent organic framework immobilized gold nanoparticles with high-efficiency catalytic performance for chemiluminescent detection of pesticide triazophos. Talanta 235:122798. https://doi.org/10.1016/j.talanta.2021.122798
Yang CJ, Yu HL, Hu XF, Chen HL, Wu H, Deng CH, Sun NR (2021) Gold-Doped covalent-organic framework reveals specific serum metabolic fingerprints as point of Crohn’s disease diagnosis. Adv Funct Mater 31:2105478. https://doi.org/10.1002/adfm.202105478
Jiang B, Qu YY, Zhang LH, Liang Z, Zhang YK (2016) 4-Mercaptophenylboronic acid functionalized graphene oxide composites: preparation, characterization and selective enrichment of glycopeptides. Anal Chim Acta 912:41–48. https://doi.org/10.1016/j.aca.2016.01.018
Liu HL, Lian B (2018) A guanidyl-functionalized TiO2 nanoparticle-anchored graphene nanohybrid for enhanced capture of phosphopeptides. RSC Adv 8:29476–29481. https://doi.org/10.1039/c8ra90074d
Li JY, Zhang S, Gao W, Hua Y, Lian HZ (2020) Guanidyl-Functionalized magnetic bimetallic MOF nanocomposites developed for selective enrichment of phosphopeptides. ACS Sustainable Chem Eng 8:16422–16429. https://doi.org/10.1021/acssuschemeng.0c04118
Zhang N, Sun NR, Deng CH (2021) Rapid isolation and proteome analysis of urinary exosome based on double interactions of Fe3O4@TiO2-DNA aptamer. Talanta 221:121571. https://doi.org/10.1016/j.talanta.2020.121571
Smolarz M, Pietrowska M, Matysiak N, Mielańczyk Ł, Widłak P (2019) Proteome profiling of exosomes purified from a small amount of human serum: the problem of co-purified serum components. Proteomes 7(2):18. https://doi.org/10.3390/proteomes7020018
Funding
This work is supported by National Natural Science Foundation of China (21927805), Natural Science Foundation of Zhejiang Province (LY22B050008), Major Science and Technology Projects in Ningbo (2020Z090), and the K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, B., Wang, B., Feng, Q. et al. Magnetic guanidyl–functionalized covalent organic framework composite: a platform for specific capture and isolation of phosphopeptides and exosomes. Microchim Acta 189, 330 (2022). https://doi.org/10.1007/s00604-022-05394-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05394-3