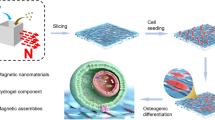
Abstract
Background
Fe3O4 nanoparticles are highly desired for constructing endogenous magnetic microenvironment in scaffold to accelerate bone regeneration due to their superior magnetism. However, their random arrangement easily leads to mutual consumption of magnetic poles, thereby weakening the magnetic stimulation effect.
Methods
In this study, magnetic nanochains are synthesized by magnetic-field-guided interface co-assembly of Fe3O4 nanoparticles. In detail, multiple Fe3O4 nanoparticles are aligned along the direction of magnetic force lines and are connected in series to form nanochain structures under an external magnetic field. Subsequently, the nanochain structures are covered and fixed by depositing a thin layer of silica (SiO2), and consequently forming linear magnetic nanochains (Fe3O4@SiO2). The Fe3O4@SiO2 nanochains are then incorporated into poly l-lactic acid (PLLA) scaffold prepared by selective laser sintering technology.
Results
The results show that the Fe3O4@SiO2 nanochains with unique core–shell structure are successfully constructed. Meanwhile, the orderly assembly of nanoparticles in the Fe3O4@SiO2 nanochains enable to form magnetic energy coupling and obtain a highly magnetic micro-field. The in vitro tests indicate that the PLLA/Fe3O4@SiO2 scaffolds exhibit superior capacity in enhancing cell activity, improving osteogenesis-related gene expressions, and inducing cell mineralization compared with PLLA and PLLA/Fe3O4 scaffolds.
Conclusion
In short, the Fe3O4@SiO2 nanochains endow scaffolds with good magnetism and cytocompatibility, which have great potential in accelerating bone repair.
Similar content being viewed by others
Introduction
Recent scaffolds lack the capacity to effectively modulate cell growth or tissue reconstruction, resulting in slow bone regeneration and even failure of bone implantation [1,2,3,4]. It is well known that cells are magnetically sensitive due to the diamagnetism of cell membranes [5], and exposure to magnetic fields served to alter membrane flux and regulate ion channels and biochemical pathways [2f-i). Particularly, the distribution of Si and Fe elements further confirmed the shell-core structure of the nanochains. The results confirmed that the Fe3O4 nanoparticles could be induced to align in a nanochain though magnetic dipolar interaction under external magnetic field.
Magnetic field distribution around single Fe3O4 nanoparticle and nanochain in the same direction of external magnetic field was analyzed using finite element method (COMSOL Multiphysics), as shown in Fig. 3a-c. It could be seen that the magnetic dipole moment of a Fe3O4 nanoparticle reached a saturated value under the adequately strong magnetic field (Fig. 3a). When the centerlines of two adjacent nanoparticles were aligned with the direction of the external magnetic field, the dipole–dipole interaction was attractive (Fig. 3b). In the case of the interaction energy was large enough to overcome thermal fluctuations, the magnetic dipole–dipole force drove the self-assembly of nanoparticles into nanochain along the dipole moment (Fig. 3c). In this condition, the dipole–dipole coupling reached the maximum, and the magnetism of nanochain could be regarded as the magnetic energy coupling among multiple nanoparticles. The magnetic strength of nanochain was higher than that of randomly arranged Fe3O4 nanoparticles.
a Magnetic field distribution around a Fe3O4 nanoparticle. The attractive (b) dipole–dipole forces between two adjacent nanoparticles drive the formation of nanochains along the magnetic field (c). d Magnetization curves, (e) XPS spectra of Fe3O4@SiO2 nanochains. (e1) XPS survey spectrum along with the spectra of (e2) Fe2p, (e3) Si2p and (e4) O1s. (f) XRD patterns, (g) FTIR spectra
The magnetic properties of Fe3O4 nanoparticles and Fe3O4@SiO2 nanochains were detected and presented in Fig. 3d. It could be seen that both nanoparticles and nanochains exhibited superior magnetism, which was an important advantage for their applicability in biomedicine [22, 23]. The saturation magnetization of Fe3O4 was 52.2 emu/g. The relatively low saturation magnetization of Fe3O4@SiO2 was due to the introduction of non-magnetic SiO2 shells decreased the weight ratio of Fe3O4 in Fe3O4@SiO2. This phenomenon was also discovered by M. Tarhini and A. Bitar et al. [24, 25].
XPS spectra of Fe3O4@SiO2 nanochains were presented in Fig. 3e. The typical Fe2p, O1s and Si2p peaks were clearly observed (Fig. 3 e1). In detail, the peaks centered at 711.4, 723.0 and 726.0 eV were respectively corresponded to Fe2+2p3/2, Fe2+2p1/2 and Fe3+2p1/2 of Fe3O4 (Fig. 3 e2), while the peaks centered at 102.7 and 104.2 eV were assigned to Si–OH and Si–O-Si of SiO2 (Fig. 3 e3) [26]. It was worth noting that the binding energy of O1s was 533.1 eV (Fig. 3 e4), which was higher than that of Fe3O4 (529.6 eV) by 3.5 eV. This was mainly due to the formation of Fe–O-Si chemical bond (530.2 eV) decreased the electronic density of O binding Fe, resulting in the chemical shift of binding energy of O1s. Moreover, the coexistence of Si–O-Si and Fe–O-Si verified the coating of SiO2 on the nanochains.
The XRD patterns of Fe3O4 nanoparticles and Fe3O4@SiO2 nanochains were exhibited in Fig. 3f. There were typical diffraction peaks of (200), (311), (400), (422), (511) and (440) planes, which corresponded to Fe3O4 presented in both patterns, confirming that the crystal structure of Fe3O4 nanoparticles were completely preserved during the synthesis of Fe3O4@SiO2. Compared with Fe3O4, a new broad diffraction at around 20° appeared in Fe3O4@SiO2, which was attributed to the amorphous SiO2 shell [27]. In the FTIR spectrum (Fig. 3g), the absorption peak at 1076 cm−1 in Fe3O4@SiO2 was adscripted to Si–O bond while the peak at 592 cm−1 was attributed to Fe–O bond [28,29,30], which further confirmed that the core–shell structure of Fe3O4@SiO2 nanochains.
Physical and chemical properties
The porous scaffold with honeycomb structure was shown in Fig. 4a. The pore size of the scaffold was 800 ± 50 μm, which was proven to be beneficial to cell adhesion and climbing growth [31, 32]. The phase composition of the scaffolds was assessed using XRD (Fig. 4b). It can be clearly observed that the diffraction peaks belonged to (010), (110), (203) and (205) planes of PLLA [33]. By contrast, the new diffraction peaks corresponding to (311), (400), (551) and (440) crystal planes confirmed the spinel structure of Fe3O4 in PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds [34].
a Optical graphs of SLS prepared honeycomb scaffold. b XRD patterns. c Magnetization curves. d Magnetization behaviors at magnetic field from -90 to 90 Oe. e Compressive strength and compressive modulus (n = 5, *p < 0.05), (f) tensile stress–strain curves, (g) TGA curves and (h) hydrophilicity of PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds
The magnetic behaviors of the PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds were shown in Fig. 4c and d. It was clearly seen that the introduction of Fe3O4 and Fe3O4@SiO2 endowed the non-magnetic PLLA scaffold favorable magnetic properties. This was conducive to construct magnetic microenvironment in scaffold, which was expected to enhance cell viability and promote cell growth through magnetic stimulation.
Mechanical properties of the scaffolds were evaluated via compressive and tensile tests, with the results shown in Fig. 4e and f. The compressive strength and modulus of PLLA scaffolds were only 22.3 ± 0.9 MPa and 78.4 ± 6.2 MPa. Encouragingly, the compressive strength and modulus of the PLLA/Fe3O4@SiO2 scaffolds were 41.8 ± 1.6 MPa and 142.6 ± 8.5 MPa, which were increased by 87.4% and 80.6% compared with PLLA scaffolds. Moreover, the PLLA/Fe3O4@SiO2 scaffolds also exhibited much higher the tensile strength and strain than those of PLLA and PLLA/Fe3O4 scaffolds, which were 13.34 ± 1.2 MPa and 2.13%, respectively. The high mechanical properties of PLLA/Fe3O4@SiO2 scaffolds were attributed to that the Fe3O4@SiO2 nanochains acted as rigid reinforcement to enhance the stress transfer efficiency in the matrix.
The TGA measurement were performed to analyze the thermal decomposition and the corresponding residual weight of scaffolds (Fig. 4g). The slight weight loss below 300 °C of the scaffolds was related to the evaporation of adsorbed water molecules. Obviously, the thermal decomposition temperature of PLLA scaffolds was about 300 ~ 400 °C, while the range of decomposition temperature leftward shifted and narrowed after adding Fe3O4 and Fe3O4@SiO2. This confirmed that the addition of Fe3O4 and Fe3O4@SiO2 catalyzed the thermal decomposition of PLLA. Additionally, the residual weight of the PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds was 7.4 wt.% and 7.6 wt.%, respectively, which was close to the nominal content (8 wt.%) of Fe3O4 and Fe3O4@SiO2 introduced into PLLA matrix.
Generally, a scaffold with favorable hydrophilicity is more conducive to cell adhesion [35]. The hydrophilicity of PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds was investigated via water contact angle test. As shown in Fig. 4h, the contact angle on the PLLA scaffold was 86.2 ± 2.8°. By contrast, the contact angle decreased after the adding Fe3O4 nanoparticles, indicating the improvement of hydrophilicity. This could be attributed to the presence of hydroxyl groups on the Fe3O4 nanoparticles in aqueous environment. Moreover, the PLLA/Fe3O4@SiO2 scaffolds exhibited the best hydrophilicity, which was mainly due to the silanol groups of SiO2 absorbed water molecule via hydrogen bonding.
Cytocompatibility
The cytocompatibility of scaffolds is a necessary and crucial element in the bone repair process because it determines whether cells can adhere, grow, and proliferate on the scaffold [36, 37]. Herein, the cytocompatibility of the PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds were assessed. As shown in Fig. 5a, cells adhered well on all scaffolds, indicating that the PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds had good biocompatibility. Particularly, better cell adhesion morphology displayed on PLLA/Fe3O4@SiO2 scaffold than that on PLLA and PLLA/Fe3O4 scaffolds at the same time point. Moreover, the cells completely expanded and essentially presented normal topological configuration on PLLA/Fe3O4@SiO2 scaffold after 7 days of cultivation, indicating that the Fe3O4@SiO2 nanochains in scaffold were more conducive to cell adhesion and expansion.
Cell viability is also an important indicator for evaluating the cytocompatibility of the scaffold [38]. To investigate the cells viability induced by the PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds, the cells were strained with calcein AM. Normally, calcein AM only stains living cells, because calcein AM as a dye can be transformed into a membrane impermeable fluorescent analogue by the cell esterases, and the fluorescence will leak out when the cell membrane is completely damaged [39, 40]. As shown in Fig. 5b, the density of living cells in PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffold groups was significantly enhanced with time, confirming that all scaffolds possessed the ability to enhance cell activity. Notably, the cells increased exponentially from 3 to 7 days with the highest density observing in the cells which cocultured with PLLA/Fe3O4@SiO2 scaffold, indicating that the Fe3O4@SiO2 nanochains in scaffold significantly enhanced cell viability and promoted cell proliferation.
Cell proliferation is one of the important physiological functions of living cells. To quantitatively study the cell proliferation capacity on the PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds, the CCK-8 assay was carried out (Fig. 5c). It could be clearly seen that the optical density (OD) value of cells on all the scaffolds increased significantly with incubating time. Compared with the PLLA scaffold, higher OD value of cells presented on the PLLA/Fe3O4 scaffold, indicating that the Fe3O4 nanoparticles in the scaffold promoted cell proliferation. Especially, the OD value of cells on PLLA/Fe3O4@SiO2 scaffold was markedly higher than that on PLLA/Fe3O4 scaffold, indicating that the Fe3O4@SiO2 nanochains further promoted cell proliferation.
As one of the early indicators of osteogenic differentiation [41], ALP activity of cells cultured on the scaffolds for 7 days was qualitatively analyzed (Fig. 6a). It could be clearly seen that the cells cocultured with the PLLA/Fe3O4@SiO2 scaffold samples exhibited higher ALP activity than PLLA and PLLA/Fe3O4 samples. As one of the late markers of osteogenic differentiation [42], the Alizarin Red staining was performed to assess the extracellular matrix mineralization of cells cocultured with the scaffolds for 7 days (Fig. 6b). As expected, there were obvious red precipitates in all scaffold groups. It was worth noting that the mineral deposition was significantly enhanced in PLLA/Fe3O4@SiO2 samples compared to other groups, mainly due to the stronger magnetic stimulation effect of Fe3O4@SiO2 nanochains. The above results demonstrated that the Fe3O4@SiO2 nanochains in scaffold markedly enhanced cell activity and promoted cell proliferation, differentiation, and mineralization.
The bone-related gene expressions including RUNX2, OPN, OCN and OSX on PLLA, PLLA/Fe3O4 and PLLA/Fe3O4@SiO2 scaffolds were investigated (Fig. 7). From an overall perspective, the expression levels of RUNX2, OPN, OCN and OSX on day 7 were greatly higher than on day 3. Especially, the expression level of them on the PLLA/Fe3O4@SiO2 scaffold were markedly higher than that on PLLA/Fe3O4 and PLLA scaffolds at any time. The results showed that the Fe3O4@SiO2 nanochains provided a more favorable magnetic microenvironment for cell differentiation than Fe3O4 nanoparticles in scaffolds, confirming that the superior capability of Fe3O4@SiO2 nanochains to promote cell differentiation.
Discussion
It is well known that various cells, such as mesenchymal stem cells, osteoblasts, and endothelial cells are magnetically sensitive due to the diamagnetism of cell membranes [43]. Inspired by these, researchers have applied different external magnetic fields to study the roles of magnetic stimulation in bone repair in recent years [44, 45]. It was found that the external magnetic fields could induce a series of cell behaviors by regulating cell surface receptors and signaling pathways via magnetic stimulations, thereby accelerating new bone regeneration or inhibiting osteoclast resorption. However, the need of magnetic field generators limits the clinical application of magnetic stimulation to a certain extent.
To solve the above problem, it would be an effective means to construct an endogenous magnetic microenvironment in bone scaffolds by introducing magnetic materials. As a highly biocompatible and magnetic materials, Fe3O4 nanoparticles have received clinical approval from the Food and Drug Administration. The scaffolds loaded with Fe3O4 nanoparticles indeed effectively enhanced cell viability and promoted cell proliferation [46, 47]. However, the random arrangement of Fe3O4 nanoparticles in the scaffolds greatly compromised their positive magnetic stimulation effects, due to the mutual repulsive between adjacent magnetic dipoles.
In present study, we constructed Fe3O4@SiO2 nanochains with uniform shell-core structure by magnetic-field-guided interface co-assembly of Fe3O4 nanoparticles (Fig. 2). The simulation analysis results of magnetic field distribution proved the orderly assembly of Fe3O4 nanoparticles in the Fe3O4@SiO2 nanochains formed magnetic energy coupling and obtained a highly magnetic micro-field (Fig. 3a-c). The results are consistent with the analysis of Yin Yadong's team [48,49,50]. From the results of magnetic tests, the Fe3O4@SiO2 nanochains still preserved the superior superparamagnetism of Fe3O4 nanoparticles. The good magnetism and high surface areas endowed Fe3O4@SiO2 nanochains with great potential for use in biomedicine.
To better understand the biological advantages of Fe3O4@SiO2 nanochains in scaffolds, a series of in vitro cell experiments were performed. Compared to PLLA/Fe3O4 scaffolds, PLLA/Fe3O4@SiO2 scaffolds are more conducive to cell adhesion and expansion, especially further enhancing cell viability, proliferation, differentiation, mineralization, and bone-related gene expressions (Figs. 5, 6 and 7). It could be attributed to the stronger magnetic stimulation effect of Fe3O4@SiO2 nanochains. In terms of mechanism, the orderly assembly of Fe3O4 nanoparticles obtained magnetic energy coupling, resulting in a highly micro-field that stimulated the surrounding cells to respond (Fig. 8). In this case, the membrane flux of the diamagnetic cell membrane would be modified. Moreover, the strong magnetic singles would activate receptors on the cell membrane, thereby modulating a series of signaling pathways including Ca2+ channels, mitogen-activated protein kinase (MAPK), bone morphogenetic protein-2 (BMP-2) and integrins [6, 10, 41, 51]. Then, the corresponding downstream transcription factors were regulated, and consequently osteogenesis-related gene expressions of RUNX2, OPN, OCN and OSX were up-regulated. Hence, the Fe3O4@SiO2 nanochains in scaffold possessed great potential in accelerating bone repair.
Conclusions
A magnetic-field-guided interface co-assembly of Fe3O4 nanoparticles had been demonstrated to rationally synthesis unique Fe3O4@SiO2 nanochains. The obtained Fe3O4@SiO2 nanochains exhibited high magnetic susceptibility and excellent magnetic induction intensity. Importantly, the superior magnetic properties of nanochains enhanced the interaction between PLLA/Fe3O4@SiO2 scaffold and cells. As a result, the nanochains in scaffold effectively enhanced cell activity, proliferation, differentiation, and mineralization as well as bone-related gene expressions. These findings confirmed the superparamagnetic scaffold incorporated with Fe3O4@SiO2 nanochains could accelerate the repair of bone defect.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PLLA:
-
Poly l-lactic acid
- SiO2 :
-
Silica
- SLS:
-
Selective laser sintering
- TEOS:
-
Tetraothorsilicate
- TEM:
-
Transmission electron microscope
- XPS:
-
X-ray photoelectron spectrometer
- FTIR:
-
Fourier transform infra-red spectrometer
- XRD:
-
X-ray diffractometer
- VSM:
-
Vibrating sample magnetometer
- DMEM:
-
Dulbecco's Modified Eagle's Medium
- SEM:
-
Scanning electron microscope
- CCK-8:
-
Cell Counting Kit-8
- ALP:
-
Alkaline phosphatase
- Runx2:
-
Runt-related transcription factor-2
- OPN:
-
Osteopontin
- OCN:
-
Osteocalcin
- OSX:
-
Osterix
References
Zhang M, Lin R, Wang X, Xue J, Deng C, Feng C, et al. 3D printing of Haversian bone-mimicking scaffolds for multicellular delivery in bone regeneration. Science Adv. 2020;6:eaa6725.
Othman Z, Fernandes H, Groot AJ, Luider TM, Alcinesio A, de Melo PD, et al. The role of ENPP1/PC-1 in osteoinduction by calcium phosphate ceramics. Biomaterials. 2019;210:12–24.
Pina S, Oliveira JM, Reis RL. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: a review. Adv Mater. 2015;27:1143–69.
Qi F, Gao X, Shuai Y, Peng S, Deng Y, Yang S, et al. Magnetic-driven wireless electrical stimulation in a scaffold. Compos B Eng. 2022;237:109864.
Lew W-Z, Feng S-W, Lee S-Y, Huang H-M. The review of bioeffects of static magnetic fields on the oral tissue-derived cells and its application in regenerative medicine. Cells. 2021;10:2662.
**a Y, Sun J, Zhao L, Zhang F, Liang X-J, Guo Y, et al. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183:151–70.
Kong Y, Duan J, Liu F, Han L, Li G, Sun C, et al. Regulation of stem cell fate using nanostructure-mediated physical signals. Chem Soc Rev. 2021;50:12828–72.
Qiao Y, Liu X, Li B, Han Y, Zheng Y, Yeung KWK, et al. Treatment of MRSA-infected osteomyelitis using bacterial capturing, magnetically targeted composites with microwave-assisted bacterial killing. Nat Commun. 2020;11:1–13.
Khizar S, Ahmad NM, Zine N, Jaffrezic-Renault N, Errachid-el-salhi A, Elaissari A. Magnetic nanoparticles: From synthesis to Theranostic applications. ACS Applied Nano Materials. 2021;4(5):4284–306.
Shuai C, Yang W, He C, Peng S, Gao C, Yang Y, et al. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater Des. 2020;185:108275.
Li J, Lu X, Zhang Y, Cheng F, Li Y, Wen X, et al. Transmittance tunable smart window based on magnetically responsive 1D nanochains. ACS Appl Mater Interfaces. 2020;12:31637–44.
**ong Q, Lim CY, Ren J, Zhou J, Pu K, Chan-Park MB, et al. Magnetic nanochain integrated microfluidic biochips. Nat Commun. 2018;9:1–11.
Ma M, Zhang Q, Dou J, Zhang H, Yin D, Geng W, et al. Fabrication of one-dimensional Fe3O4/P (GMA-DVB) nanochains by magnetic-field-induced precipitation polymerization. J Colloid Interface Sci. 2012;374:339–44.
Ma M, Zhang Q, Zhang H, **n T, Zhang B, Fan X. One-pot synthesis of highly magnetically sensitive nanochains coated with a fluorescent shell by magnetic-field-induced precipitation polymerization. Sci Adv Mater. 2013;5:623–9.
Sun J, Liu X, Huang J, Song L, Chen Z, Liu H, et al. Magnetic assembly-mediated enhancement of differentiation of mouse bone marrow cells cultured on magnetic colloidal assemblies. Sci Rep. 2014;4:1–8.
Zhou J, Wang C, Wang P, Messersmith PB, Duan H. Multifunctional magnetic nanochains: exploiting self-polymerization and versatile reactivity of mussel-inspired polydopamine. Chem Mater. 2015;27:3071–6.
Jia J, Yu JC, Wang Y-XJ, Chan KM. Magnetic nanochains of FeNi3 prepared by a template-free microwave-hydrothermal method. ACS Appl Mat Interfaces. 2010;2:2579–84.
Li X, Sun L, Wang H, **e K, Long Q, Lai X, et al. Synthesis of cobalt nanowires in aqueous solution under an external magnetic field. Beilstein J Nanotechnol. 2016;7:990–4.
Wan L, Song H, Chen X, Zhang Y, Yue Q, Pan P, et al. A magnetic-field guided interface coassembly approach to magnetic mesoporous silica nanochains for osteoclast-targeted inhibition and heterogeneous nanocatalysis. Adv Mater. 2018;30(25):1707515.
Qi F, Liao R, Shuai Y, Pan H, Qian G, Peng S, et al. A conductive network enhances nerve cell response. Addit Manufact. 2022;52:102694.
Shuai C, Wang Z, Peng S, Shuai Y, Chen Y, Zeng D, et al. Water-responsive shape memory thermoplastic polyurethane scaffold triggered at body temperature for bone defect repair. Mater Chem Front. 2022;6:1456–69.
Kralj S, Makovec D. Magnetic assembly of superparamagnetic iron oxide nanoparticle clusters into nanochains and nanobundles. ACS Nano. 2015;9:9700–7.
Kralj S, Potrc T, Kocbek P, Marchesan S, Makovec D. Design and fabrication of magnetically responsive nanocarriers for drug delivery. Curr Med Chem. 2017;24:454–69.
Tarhini M, Vega-Chacón J, Jafelicci M, Zine N, Errachid A, Fessi H, et al. Structured magnetic core/silica internal shell layer and protein out layer shell (BSA@SiO2@SME): preparation and characterization. Chemistry Africa. 2020;3:127–34.
Bitar A, Vega-Chacón J, Lgourna Z, Fessi H, Jafelicci M Jr, Elaissari A. Submicron silica shell-magnetic core preparation and characterization. Colloids Surf, A. 2018;537:318–24.
Rajan A, Sharma M, Sahu NK. Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci Rep. 2020;10:1–15.
Wang L, Shen C, Cao Y. PVP modified Fe3O4@SiO2 nanoparticles as a new adsorbent for hydrophobic substances. J Phys Chem Solids. 2019;133:28–34.
Chen Z, Wang H, Wang Y, Lv R, Yang X, Wang J, et al. Improved optical damage threshold graphene Oxide/SiO2 absorber fabricated by sol-gel technique for mode-locked erbium-doped fiber lasers. Carbon. 2019;144:737–44.
Chen J, Qiao M, Wang W, Zhang Q. A novel magnetic mesoporous Fe3O4@Void@mSiO2-Pd(0) nanochains with high heterogeneous catalysis efficiency for Suzuki coupling reaction. Compos Commun. 2019;16:41–9.
Feng P, Jia J, Peng S, Shuai Y, Pan H, Bai X, et al. Transcrystalline growth of PLLA on carbon fiber grafted with nano-SiO2 towards boosting interfacial bonding in bone scaffold. Biomater Res. 2022;26:1–15.
Qi F, Wang Z, Shuai Y, Peng S, Shuai C. Sr2+ Sustained Release System Augments Bioactivity of Polymer Scaffold. ACS Appl Polymer Mater. 2022;4:2691–702.
Yang Y, Zan J, Shuai Y, Yang L, Zhang L, Zhang H, et al. In Situ growth of a metal-organic framework on graphene oxide for the chemo-photothermal therapy of bacterial infection in bone repair. ACS Appl Mater Interfaces. 2022;19:21996–2005.
Beltrán F, De La Orden M, Lorenzo V, Pérez E, Cerrada M, Urreaga JM. Water-induced structural changes in poly (lactic acid) and PLLA-clay nanocomposites. Polymer. 2016;107:211–22.
Yang L, Zou P, Cao J, Sun Y, Han D, Yang S, et al. Facile synthesis and paramagnetic properties of Fe3O4@SiO2 core-shell nanoparticles. Superlattices Microstruct. 2014;76:205–12.
Augustine R, Dan P, Schlachet I, Rouxel D, Menu P, Sosnik A. Chitosan ascorbate hydrogel improves water uptake capacity and cell adhesion of electrospun poly (epsilon-caprolactone) membranes. Int J Pharm. 2019;559:420–6.
Yang M, Shuai Y, Yang Y, Zeng D, Peng S, Tian Z, et al. In situ grown rare earth lanthanum on carbon nanofibre for interfacial reinforcement in Zn implants. Virtual Phys Prototyp. 2022;17:1–18.
Deng F, Wu P, Qian G, Shuai Y, Zhang L, Peng S, et al. Silver-decorated black phosphorus: a synergistic antibacterial strategy. Nanotechnology. 2022;33:245708.
Shuai C, Yuan X, Shuai Y, Qian G, Yao J, Xu W, et al. Nitrogen-doped carbon-ZnO heterojunction derived from ZIF-8: a photocatalytic antibacterial strategy for scaffold. Mater Today Nano. 2022;18:1002105.
Bhattacharyya S, Ghosh SS. Transmembrane TNFα-expressed macrophage membrane-coated chitosan nanoparticles as cancer therapeutics. ACS Omega. 2020;5:1572–80.
Zhang Y, Liu S, Yao Y, Chen Y, Zhou S, Yang X, et al. Invasion and defense interactions between enzyme-active liquid coacervate protocells and living cells. Small. 2020;16:2002073.
Chen H, Sun J, Wang Z, Zhou Y, Lou Z, Chen B, et al. Magnetic cell-scaffold interface constructed by superparamagnetic IONP enhanced osteogenesis of adipose-derived stem cells. ACS Appl Mater Interfaces. 2018;10:44279–89.
Wang H, Zeng X, Pang L, Wang H, Lin B, Deng Z, et al. Integrative treatment of anti-tumor/bone repair by combination of MoS2 nanosheets with 3D printed bioactive borosilicate glass scaffolds. Chem Eng J. 2020;396:125081.
Yang J, Zhang H, Shang P. Effect of static magnetic field on bone and its molecular mechanism. Chin Sci Bull. 2020;65:1238–50.
Xu H-Y, Gu N. Magnetic responsive scaffolds and magnetic fields in bone repair and regeneration. Front Mater Sci. 2014;8:20–31.
Zan J, Qian G, Deng F, Zhang J, Zeng Z, Peng S, et al. Dilemma and breakthrough of biodegradable poly-l-lactic acid in bone tissue repair. J Mater Res Technol. 2022;17(2369):2387.
Anjaneyulu U, Priyadarshini B, Vijayalakshmi U. Preparation of Ag doped hydroxyapatite-Fe3O4-chitosan composites: In vitro biocompatibility study on MG-63 cells for orthopedic applications. Adv Sci Lett. 2018;24:5901–6.
Wu ZC, Li WP, Luo CH, Su CH, Yeh CS. Rattle-Type Fe3O4@CuS Developed to Conduct Magnetically Guided Photoinduced Hyperthermia at First and Second NIR Biological Windows. Adv Func Mater. 2015;25:6527–37.
Li Z, Yang F, Yin Y. Smart materials by nanoscale magnetic assembly. Adv Func Mater. 2020;30:1903467.
Li Z, Wang M, Zhang X, Wang D, Xu W, Yin Y. Magnetic assembly of nanocubes for orientation-dependent photonic responses. Nano Lett. 2019;19:6673–80.
Wang M, He L, Yin Y. Magnetic field guided colloidal assembly. Mater Today. 2013;16:110–6.
Zhu Y, Yang Q, Yang M, Zhan X, Lan F, He J, et al. Protein corona of magnetic hydroxyapatite scaffold improves cell proliferation via activation of mitogen-activated protein kinase signaling pathway. ACS Nano. 2017;11:3690–704.
Acknowledgements
Not applicable.
Funding
This study was supported by the following funds: (1) The Natural Science Foundation of China (52105352, 51935014, 52165043, 82072084, 81871498); (2) The Project of Jiangxi University of Science and Technology (205200100535); (3) Jiang** Peng
Contributions
Shuai CJ and Chen X contributed to acquisition, analysis of data, drafted and revised the manuscript. He CX and Qian GW contributed to analysis and interpretation of the data. Shuai Y and Peng SP contributed to performing the experiments. Deng YW and Yang WJ conceptualized the experimental research, acquired the financial support/experimental resources, validated the data and reviewed the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shuai, C., Chen, X., He, C. et al. Construction of magnetic nanochains to achieve magnetic energy coupling in scaffold. Biomater Res 26, 38 (2022). https://doi.org/10.1186/s40824-022-00278-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40824-022-00278-2