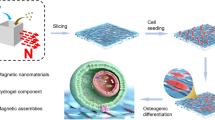
Abstract
Magnetic field has been considered to have positive effect on growth of bone. Because amagnetic nanoparticle can be regarded as one magnetic dipole, the macroscopic assemblies of magnetic nanoparticles may exhibit magnetic effect on local objects. This paper fabricated macroscopic film of γ-Fe2O3 nanoparticles by layer-by-layer (LBL) assembly on poly-D,L-lactic acid (PLA) scaffold, and studied the magnetic effect of the assembled γ-Fe2O3 nanoparticles film on primary bone marrow cells. The primary bone marrow cells were extracted from a mouse and cultured on the PLA substrate decorated by the film of γ-Fe2O3 nanoparticles after purification. Quantitative PCR (q-PCR) was used to show the cellular effect quantitatively. A just-found magnetosensing protein was employed to verify the magnetic effect of assembled film of nanoparticles on primary cells. It was exhibited that the decoration of nanoparticles enhanced themechanical property of the interface. By acting as the adhesion sites, the LBL-assembled film of nanoparticles seemed beneficial to the cellular growth and differentiation. The expression of magnetosensing protein indicated that there was magnetic effect on the cells which resulted from the assembly of magnetic nanoparticles, implying its potential as a promising interface on scaffold which can integrate the physical effect with good biocompatibility to enhance the growth and differentiation of stem cells. The LBL-assembled film of magnetic nanoparticles may boost the development of novel scaffold which can introduce the physical stimulus into local tissue in vivo.
摘要
磁场一直以来都被认为对骨生长具有促进作用. 磁性纳米颗粒可以被看作是一个磁偶极子, 因此宏观的磁性纳米颗粒组装膜也可 能对附**的物体具有磁效应. 本文通过层层自组装方法在聚乳酸支架表面制备了宏观γ-Fe2O3纳米颗粒组装膜, 研究了γ-Fe2O3纳米颗粒组 装膜对原代小鼠骨髓细胞的磁作用. 原代小鼠骨髓细胞从小鼠体内新鲜提取, 并在前述生物材料表面培养. 定量PCR用来定量表征细胞效 应, 磁场的影响通过检测一种刚刚发现的磁感应蛋白来指示. 结果表明, 表面纳米颗粒组装可以显著增**聚合物支架的力学性质, 促进细 胞生长和分化. 磁感应蛋白检测结果表明这是由于磁性纳米颗粒组装导致的磁效应引起的. 本文用磁感应蛋白证明了磁性纳米颗粒层层 自组装膜可以通过对细胞的磁效应促进干细胞的生长和分化, 该磁性纳米颗粒组装膜将会促进新一代组织工程支架的研发, 有可能将物 理刺激效应引入到体内局部组织修复中.
Similar content being viewed by others
References
Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet, 2014, 15: 82–92
Bailey AM, Mendicino M, Au P. An FDA perspective on preclinical development of cell-based regenerativemedicine products. Nat Biotechnol, 2014, 32: 721–723
Yow SZ, Quek CH, Yim EKF, et al. Collagen-based fibrous scaffold for spatial organization of encapsulated and seeded human mesenchymal stem cells. Biomaterials, 2009, 30: 1133–1142
Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater, 2009, 8: 457–470
Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res, 2014, 163: 399–408
Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotech, 2014, 32: 483–492
Ambrose CG, Clanton TO. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann Biomedical Eng, 2004, 32: 171–177
Das RK, Zouani OF. A review of the effects of the cell environment physicochemical nanoarchitecture on stem cell commitment. Biomaterials, 2014, 35: 5278–5293
Alvarado AS, Yamanaka S. Rethinking differentiation: stem cells, regeneration, and plasticity. Cell, 2014, 157: 110–119
Costa DO, Prowse PDH, Chrones T, et al. The differential regulation of osteoblast and osteoclast activity by surface topography of hydroxyapatite coatings. Biomaterials, 2013, 34: 7215–7226
Song J, Shawky JH, Kim YT, et al. Controlled surface topography regulates collective 3Dmigration by epithelial–mesenchymal composite embryonic tissues. Biomaterials, 2015, 58: 1–9
Fiedler J, Özdemir B, Bartholomä J, et al. The effect of substrate surface nanotopography on the behavior of multipotnent mesenchymal stromal cells and osteoblasts. Biomaterials, 2013, 34: 8851–8859
Isaacson BM, Bloebaum RD. Bone bioelectricity: what have we learned in the past 160 years? J Biomed Mater Res, 2010, 95A: 1270–1279
Markaki AE, Clyne TW. Magneto-mechanical stimulation of bone growth in a bonded array of ferromagnetic fibres. Biomaterials, 2004, 25: 4805–4815
Bassett CAL. Fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs). Crit Rev Biomed Eng, 1989, 17: 451–529
Reddy LH, Arias JL, Nicolas J, et al. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev, 2012, 112: 5818–5878
Sun J, Liu X, Huang J, et al. Magnetic assembly-mediated enhancement of differentiation of mouse bone marrow cells cultured on magnetic colloidal assemblies. Sci Rep, 2014, 4: 5125
Fortin JP, Wilhelm C, Servais J, et al. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc, 2007, 129: 2628–2635
Chen Z, Yin JJ, Zhou YT, et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano, 2012, 6: 4001–4012
Sarella A, Torti A, Donolato M, et al. Two-dimensional programmable manipulation of magnetic nanoparticles on-chip. Adv Mater, 2014, 26: 2384–2390
Kotov NA. Inorganic nanoparticles as protein mimics. Science, 2010, 330: 188–189
Liu X, Shimono K, Zhu LL, et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochem Biophys Res Commun, 2009, 388: 161–166
Guo Z, Hu K, Sun J, et al. Fabrication of hydrogel with cell adhesive micropatterns for mimicking the oriented tumor-associated extracellular matrix. ACS Appl Mater Interfaces, 2014, 6: 10963–10968
Hu K, Zhou N, Li Y, et al. Sliced magnetic polyacrylamide hydrogel with cell-adhesive microarray interface: a novel multicellular spheroid culturing platform. ACS Appl Mater Interfaces, 2016, 8: 15113–15119
Berman SMC, Walczak P, Bulte JWM. Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2011, 3: 343–355
Tseng P, Judy JW, Di Carlo D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat Meth, 2012, 9: 1113–1119
Fayol D, Frasca G, Le Visage C, et al. Use of magnetic forces to promote stem cell aggregation during differentiation, and cartilage tissue modeling. Adv Mater, 2013, 25: 2611–2616
Qin S, Yin H, Yang C, et al. A magnetic protein biocompass. Nat Mater, 2015, 15: 217–226
Brinkman A, Huijben M, van Zalk M, et al. Magnetic effects at the interface between non-magnetic oxides. Nat Mater, 2007, 6: 493–496
Yazyev OV, Katsnelson MI. Magnetic correlations at graphene edges: basis for novel spintronics devices. Phys Rev Lett, 2008, 100: 47209–47212
Trock DH. Electromagnetic fields and magnets. Rheumatic Disease Clinics North Am, 2000, 26: 51–62
Lednev VV. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics, 1991, 12: 71–75
Barnes FS. Some engineering models for interactions of electric and magnetic fields with biological systems. Bioelectromagnetics, 1992, 13: 67–85
Acknowledgments
This work was supported by the National Basic Research Program of China (2013CB733801) and the National Natural Science Foundation of China (21273002 and 61601227). Sun J is also thankful to the supports fromthe special fund for the top doctoral thesis of Chinese EducationMinistry (201174). Lou Z and Li Y thank the Natural Science Foundation of Jiangsu Province (BK20160939 and BK20130608). All authors are thankful to the supports from Collaborative Innovation Center of Suzhou Nano Science and Technology. Author contributions Sun J conceived and designed
Author information
Authors and Affiliations
Corresponding authors
Additional information
These two authors contributed equally to this paper.
Xuan Liu received her PhD degree in biomedical engineering from Southeast University in 2011. Now she is a lecturer at the School of Medicine, Southeast University. Her research interests include bone metabolic mechanism, osteoporosis pathogenesis and diagnostic method, bone scaffold material and bone repair.
Jie Zhang received his BSc degree in biomedical engineering from Southeast University in 2013. Now he is pursuing his master degree (biomedical engineering) at Southeast University. His research interest is the assembly of nanoparticles.
Jianfei Sun received his PhD degree in biomedical engineering from Southeast University in 2008. He is an associate professor at the School of Biological Science and Medical Engineering, Southeast University. His research interests include fabrication of nanoelectronic devices by self-assembly of nanoparticles and their application in biomedical issues.
Ning Gu received his PhD degree in biomedical engineering from the Department of Biomedical Engineering, Southeast University, Nan**g, China, in 1996. Currently he is Cheung Kong Scholar Chair Professor at the School of Biological Science and Medical Engineering, Southeast University and Director of Jiangsu Key Laboratory of Biomaterials and Devices. He has published over 300 peer-reviewed papers and obtained many awards. His research interests include application of magnetic nanomaterials in biomedicine.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, J., Tang, S. et al. Growth enhancing effect of LBL-assembled magnetic nanoparticles on primary bone marrow cells. Sci. China Mater. 59, 901–910 (2016). https://doi.org/10.1007/s40843-016-5104-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-016-5104-9