Abstract
Microbial communities are ubiquitous in nature and exhibit several attractive features, such as sophisticated metabolic capabilities and strong environment robustness. Inspired by the advantages of natural microbial consortia, diverse artificial co-cultivation systems have been metabolically constructed for biofuels, chemicals and natural products production. In these co-cultivation systems, especially genetic engineering ones can reduce the metabolic burden caused by the complex of metabolic pathway through labor division, and improve the target product production significantly. This review summarized the most up-to-dated co-cultivation systems used for biofuels, chemicals and nature products production. In addition, major challenges associated with co-cultivation systems are also presented and discussed for meeting further industrial demands.
Similar content being viewed by others
Introduction
Pure cultures dominate the current industrial bioprocesses; however, they are confronted with challenges due to the increased requirement for higher efficiency of production and fulfillment of more complicated tasks. In nature, 99% microorganisms exist in the form of microbial consortia [1]. Inspired by the omnipresent natural microbial consortia, more attention has been paid on the bioprocess development of artificial ones, which pools different engineered microorganisms in one pot [2,3,4]. However, different from natural microbial communities, which exist mainly for the survival and growth in the environment, the artificial microbial consortia are specifically constructed to broaden the scope of feedstocks, enhance the productivity of target bio-products, etc. [5,6,7].
Diverse microbial communities within the same or different species have been set up to realize more complicated tasks [8,9,10]. In addition to treatment of wastewater, biodegradation of textile azo dye and dispose of contaminated soil, recently, co-cultivation systems were also applied to produce biofuels (bioethanol, biobutanol, biodiesel, etc.), bulk chemicals (lactic acid, 2-keto-l-gulonic acid, etc.) and natural products (alkaloids, polyketides, terpenes, flavonoid, etc.) [11,12,13,14,15,16,17,18,19,20,21]. These artificial microbial consortia interact mutually through the interaction of synergism, commensalism, competition, mutualism, etc. (Fig. 1) [1]. Elaboration of the underlying mechanism in microbial communities, such as the exchange of intermediate metabolites, cell-to-cell electrical connections, communications, etc. would guide the design of artificial microbial consortia and further improve the robustness and stability of the co-cultivation systems [22,23,24,25]. Accordingly, this review summarizes the superiority of co-cultivation systems compared with pure cultures and the most updated advances in artificial microbial consortia for the production of biofuels and chemicals from renewable sources. Nevertheless, further application and development of microbial consortia are still confronted with challenges, such as the uncharacterized microbial interaction mechanisms, etc.
Advantages of co-cultivation systems over pure cultures
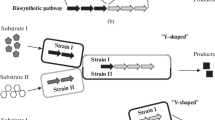
Compared with pure cultures, co-cultivation systems could broaden the substrate utilization spectra. Lignocellulose is the most abundant sustainable recourses; however, due to the complexity of cellulose-degrading systems, single strain generally can not directly utilize it to synthesize valuable products [26, 27]. In general, two common strategies were developed: one is the incorporation of target product synthesis modules into cellulolytic microbes to achieve product generation from lignocellulose; the other is the introduction of cellulase systems into product-generating microbes (Fig. 2a, b) [28, 29]. However, the long and complex pathways including cellulase secretion and/or product synthesis would burden the metabolic stress and lead to low amounts of product generated [30, 31]. On the contrary, microbial consortia offer a simpler and more efficient approach to achieve this goal through the so-called consolidated bioprocessing (CBP), in which enzymes production, substrate hydrolysis and microbial fermentation are completed in one single reactor. For example, setting up co-cultivation systems including cellulolytic Clostridium sp. and non-cellulolytic Thermoanaerobacter sp. can achieve ethanol production from cellulose through CBP. Argyros et al. [32] set up an artificial C. thermocellum–T. saccharolyticum co-cultivation system, in which organic acids formation pathways were both removed in these two constituent strains. 38 g/L of ethanol was finally produced from 92 g/L of Avicel, which was approximately 80% theoretical maximum, indicating that C. thermocellum could be a cornerstone of a robust cellulolytic platform. On the other hand, the lagged utilization of pentose in both hexose and pentose mixtures is commonly found in most microbes, known as carbon catabolic repression (CCR), when bacteria are exposed to two or more carbon sources [33]. The sequential utilization of component sugars of lignocellulose materials would reduce the whole processes efficiency. Microbial consortia enable to rationally utilize different substrates based on the specific metabolic pathway. A novel binary culture can solve the problem flexibly, in which one could only consume glucose and the other could only consume xylose, shifting the interaction modes from the competition to the commensalism [34].
Comparison between pure cultures and microbial co-culturing systems for butanol production used lignocellulose. Two strategies for achievement of butanol production from lignocellulose via CBP. a the “native cellulolytic strategy”, in which butanol synthetic pathway was introduced into cellulolytic microorganism; b the “recombinant cellulolytic strategy”, in which cellulolytic enzymes were constructed into solventogenic ones. c The strategy for microbial co-culturing systems including lignocellulolytic microorganisms and solventogenic bacteria
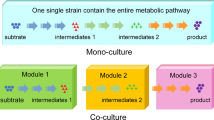
When the biosynthetic pathway of target product is long and complicated, a large number of genes would be heterologously expressed in single strains. Generally, the biochemical properties and expression levels of introduced enzymes vary to a large extent. A single host cell cannot provide the optimal environment to perform the function well for all enzymes, while microbial consortia can provide diversified cellular environments for different enzymes. Especially, when a biosynthetic pathway is composed of both prokaryotic and eukaryotic enzymes, a combination of bacterial and fungal hosts would be highly advantageous over using either host alone [35]. In addition, excessive cellular resources consumption and overwhelming metabolic burden often lead to the impaired growth and/or poor biosynthetic behavior of single host strain [36]. Microbial consortia can reduce this metabolic burden through the strategy of labor division, which not only benefits the growth of individual strains, but also improves the performance of overall bio-production (Fig. 3) [37]. Furthermore, insufficient supply of precursors or excessive accumulation of intermediate products could both influence the end-products generation. In pure culture, the relative expression level of different genes is adjusted through promoter strength, gene copy number, ribosomal binding site etc. [38]. Building microbial consortia is a straightforward way to flexibly balance the biosynthetic strength through changing strain–strain ratios [39].
In pure cultures, most strains have individual suitable conditions for the growth. If cultural conditions changed, the growth and metabolism of strains would be affected. Microbial consortia could endure more changeable environments, providing an important new frontier for industrial production [1]. In microbial consortia, environmental disturbance can be dynamically balanced and regulated due to the coordination and cooperation of different strains. The undesired interference within different pathway modules in host strains would also be reduced [40]. Modular compartmentalization offers a new effective approach to limit negative interaction between pathway modules and improve the biosynthesis performance. Hence, microbial consortia commonly possess higher stability and robustness to environmental perturbations.
Biofuels production by using co-cultivation systems
Bioethanol
As an environmentally friendly and sustainable source, biofuels production including bioethanol, biobutanol and biodiesel has gained considerable interests [41,42,43]. Bioethanol was regarded as one of the most promising biofuels, particularly as a carbon-neutral liquid transportation fuel [44]. Solventogenic yeasts, such as Saccharomyces cerevisiae and some bacteria, such as Thermoanaerobacter species are widely used to produce ethanol [45,46,47]. However, the feedstock spectrum is limited to some starchy-based materials [48]. Compared to grain-derived feedstocks, lignocellulose is a more economically feasible alternative because of its abundance and low cost [49, 50]. An artificial Escherichia coli binary culture was constructed for direct conversion of hemicellulose into ethanol. The final ethanol concentration reached 2.84 g/L, which is 55% of the theoretical yield [51]. In this binary system, one E. coli strain was engineered to hydrolyze hemicellulose to xylooligosaccharides through co-expression of two hemicellulase genes. Xylooligosaccharide-utilizing enzymes were then over-expressed in the other E. coli strain to realize the conversion of xylooligosaccharides into ethanol. This co-cultivation system distributed the metabolic burden through extracellular and intracellular expression of different functional enzymes, resulting in the improved ethanol production over pure cultures. Furthermore, cellulase system can also be built in a microbial consortium. For example, dual-microbe Bacillus/yeast system was developed for cellulosic ethanol production. Recombinant B. subtilis carries eight cellulosomal genes originating from C. thermocellum: one scaffolding protein gene (cipA), one cell-surface anchor gene (sdbA), two exo-glucosidase genes (celK and celS), two endo-glucanase genes (celA and celR), and two xylanase genes (xynC and xynZ). The partner Kluyveromyces marxianus KY3-NpaBGS carries a glucosidase (NpaBGS) gene from rumen fungus. Ultimately, 9.5 g/L of ethanol was produced from 20 g/L of cellulose (Table 1) [52].
Considering the complex of lignocellulose degradation enzymes, co-culturing cellulolytic microorganism with ethanol-producing one is a convenient and flexible approach to produce ethanol from lignocellulose through CBP. Cellulolytic C. thermocellum is a model organism for CBP; however, its application was limited due to the low ethanol yield [53,54,55]. Considering its efficient capability of cellulose degradation, C. thermocellum can be co-cultured with non-cellulolytic Thermoanaerobacter strains (X514 and 39E), which showed high efficiency of ethanol production [56]. The final ethanol production achieved at 7.56 and 6.59 g/L, respectively, which were significantly improved by 194–440%. The labor division is straightforward in this system: C. thermocellum is mainly responsible for cellulolysis, while Thermoanaerobacter sp. takes charge of ethanol production owing to its high-efficient ethanol production capability. The interaction within these two strains was through the exchange of intermediate metabolites. Similarly, a co-cultivation system, in which cellulose hydrolysis and ethanol production were conducted by C. phytofermentans and S. cerevisiae, was set up [ In recent years, construction of co-cultivation systems for biofuels and chemicals production has attracted more and more attention. Not only limited to simply mix the wild strains, co-cultivation has also expanded into synthetic biology. The introduction of synthetic intercellular communication into the cell engineering toolbox will open new frontiers and greatly contribute to the future success of synthetic biology and its applications. Although the production could be improved when using co-cultivation systems, challenges still exist. Currently, studies associated with co-cultivation systems are mainly constricted at the levels of exchange of intermediate metabolites. Other elements of environmental variation, such as energy flux, signal exchange and nutrient cycling are still unknown. Only based on the comprehensive understanding of the interaction among microbes, the improvement of robustness, stability and reproducibility can be further achieved.Conclusions
Availability of data and materials
Not applicable.
Abbreviations
- CBP:
-
consolidated bioprocessing
- CCR:
-
carbon catabolic repression
- ABE:
-
acetone–butanol–ethanol
- AECC:
-
alkali extracted corn cobs
- MA:
-
muconic acid
- DHS:
-
3-dehydroshikimic acid
- 2-KGA:
-
2-keto-l-gulonic acid
- NP:
-
natural product
References
Ding MZ, Song H, Wang EX, Liu Y, Yuan YJ. Design and construction of synthetic microbial consortia in China. Synth Syst Biotechnol. 2016;1(4):230–5.
Jiang YJ, Zhang T, Lu JS, Dürre P, Zhang WM, Dong WL, Zhou J, Jiang M, **n FX. Microbial co-culturing systems: butanol production from organic wastes through consolidated bioprocessing. Appl Microbiol Biotechnol. 2018;102(13):5419–25.
Lindemann SR, Bernstein HC, Song HS, Fredrickson JK, Fields MW, Shou WY, Johnson DR, Beliaev AS. Engineering microbial consortia for controllable outputs. ISME J. 2016;10(9):2077–84.
Minty JJ, Singer ME, Scholz SA, Bae CH, Ahn JH, Foster CE, Liao JC, Lin XN. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc Natl Acad Sci USA. 2013;110(36):14592–7.
Bhatia SK, Yoon JJ, Kim HJ, Hong JW, Hong YG, Song HS, Moon YM, Jeon JM, Kim YG, Yang YH. Engineering of artificial microbial consortia of ralstonia eutropha and bacillus subtilis for poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production from sugarcane sugar without precursor feeding. Bioresour Technol. 2018;257:92–101.
Diender M, Stams AJM, Sousa DZ. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol Biofuels. 2016;9(1):82.
Jiang LL, Liu HF, Mu Y, Sun YQ, **u ZL. High tolerance to glycerol and high production of 1,3-propanediol in batch fermentations by microbial consortium from marine sludge. Eng Life Sci. 2017;17(6):635–44.
Zuck KM, Shipley S, Newman DJ. Induced production of n-formyl alkaloids from Aspergillus fumigatus by co-culture with Streptomyces peucetius. J Nat Prod. 2011;74(7):1653–7.
Dürre P. Butanol formation from gaseous substrates. FEMS Microbiol Lett. 2016;363(6):fnw40. https://doi.org/10.1093/femsle/fnw040.
Nakayama S, Kiyoshi K, Kadokura T, Nakazato A. Butanol production from crystalline cellulose by co-cultured Clostridium thermocellum and Clostridium saccharoperbutylacetonicum N1-4. Appl Environ Microbiol. 2011;77(18):6470–5.
He F, Hu W, Li Y. Biodegradation mechanisms and kinetics of azo dye 4BS by a microbial consortium. Chemosphere. 2004;57(4):293–301.
Khouni I, Marrot B, Amar RB. Treatment of reconstituted textile wastewater containing a reactive dye in an aerobic sequencing batch reactor using a novel bacterial consortium. Sep Purif Technol. 2012;87:110–9.
Safonova E, Kvitko KV, Iankevitch MI, Surgko LF, Afti IA, Reisser W. Biotreatment of industrial wastewater by selected algal-bacterial consortia. Eng Life Sci. 2004;4(4):347–53.
Xu XH, Liu XM, Zhang L, Mu Y, Zhu XY, Fang JY, Li SP, Jiang JD. Bioaugmentation of chlorothalonil-contaminated soil with hydrolytically or reductively dehalogenating strain and its effect on soil microbial community. J Hazard Mater. 2018;351:240–9.
Sabra W, Dietz D, Tjahjasari D, Zeng AP. Biosystems analysis and engineering of microbial consortia for industrial biotechnology. Eng Life Sci. 2010;10(5):407–21.
Bertrand S, Bohni N, Schnee S, Schumpp O, Gindro K, Wolfender JL. Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol Adv. 2014;32(6):1180–204.
Eiteman MA, Lee SA, Altman R, Altman E. A substrate-selective co-fermentation strategy with Escherichia coli produces lactate by simultaneously consuming xylose and glucose. Biotechnol Bioeng. 2009;102(3):822–7.
Wang EX, Ding MZ, Ma Q, Dong XT, Yuan YJ. Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb Cell Fact. 2016;15(1):21.
Schroeckh V, Scherlach K, Nützmann HW, Shelest E, Schmidtheck W, Schuemann J, Martin K, Hertweck C, Brakhage AA. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc Natl Acad Sci USA. 2009;106(34):14558–63.
**n FX, He JZ. Characterization of a thermostable xylanase from a newly isolated Kluyvera species and its application for biobutanol production. Bioresour Technol. 2013;135(2):309–15.
Tsai SL, Goyal G, Chen W. Surface display of a functional minicellulosome by intracellular complementation using a synthetic yeast consortium and its application to cellulose hydrolysis and ethanol production. Appl Environ Microbiol. 2010;76(22):7514–20.
Lovley DR. Happy together: microbial communities that hook up to swap electrons. ISME J. 2016;11(2):327–36.
Fischbach MA, Segre JA. Signaling in host-associated microbial communities. Cell. 2016;164(6):1288–300.
Kenny DJ, Balskus EP. Engineering chemical interactions in microbial communities. Chem Soc Rev. 2018;47(5):1705–29.
Brenner K, You L, Arnold FH. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008;26(9):483–9.
Deng AJ, Ren JL, Wang WJ, Li HL, Lin QX, Yan YH, Sun RC, Liu GL. Production of xylo-sugars from corncob by oxalic acid-assisted ball milling and microwave-induced hydrothermal treatments. Ind. Crop. Prod. 2016;79:137–45.
Li HL, Chen XF, Ren JL, Deng H, Peng F, Sun RC. Functional relationship of furfural yields and the hemicellulose-derived sugars in the hydrolysates from corncob by microwave-assisted hydrothermal pretreatment. Biotechnol Biofuels. 2015;8:127–39.
Olson DG, McBride JE, Shaw AJ, Lynd LR. Recent progress in consolidated bioprocessing. Curr Opin Biotech. 2012;23(3):396–405.
Yang X, Xu M, Yang ST. Metabolic and process engineering of Clostridium cellulovorans for biofuel production from cellulose. Metab Eng. 2015;32:39–48.
Shanmugam S, Hari A, Ulaganathan P, Yang F, Krishnaswamy S, Wu YR. Potential of biohydrogen generation using the delignified lignocellulosic biomass by a newly identified thermostable laccase from Trichoderma asperellum strain BPLMBT1. Int J Hydrogen Energy. 2018;43(7):3618–28.
Mingardon F, Chanal A, Tardif C, Fierobe H. The issue of secretion in heterologous expression of Clostridium cellulolyticum cellulase-encoding genes in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 2011;77(9):2831–8.
Argyros DA, Tripathi SA, Barrett TF, Rogers SR, Feinberg LF, Olson DG, Foden JM, Miller BB, Lynd LR, Hogsett DA. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl Environ Microbiol. 2011;77(23):8288–94.
Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008;11(2):87–93.
Zhang HR, Pereira B, Li ZJ, Stephanopoulos G. Engineering Escherichia coli co-culture systems for the production of biochemical products. Proc Natl Acad Sci USA. 2015;112(27):8266–71.
Zhou K, Qiao K, Steven E, Gregory S. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol. 2015;33(4):377–83.
Wu G, Yan Q, Jones JA, Tang YJ, Fong SS, Koffas MA. Metabolic burden: cornerstones in synthetic biology and metabolic engineering applications. Trends Biotechnol. 2016;34:652–64.
Agapakis CM, Boyle PM, Silver PA. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat Chem Biol. 2012;8(6):527–35.
Jones JA, Toparlak ÖD, Koffas MA. Metabolic pathway balancing and its role in the production of biofuels and chemicals. Curr Opin Biotechnol. 2015;33:52–9.
Jones JA, Koffas MA. Optimizing metabolic pathways for the improved production of natural products. Methods Enzymol. 2016;575:179–93.
Zhang H, Wang X. Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng. 2016;37:114–21.
Jiang YJ, **n FX, Lu JS, Dong WL, Zhang WM, Zhang M, Wu H, Ma JF, Jiang M. State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour Technol. 2017;245:1498–506.
Zhang J, Wang MY, Gao MT, Fang X, Yano S, Qin SL, **a RR. Efficient acetone–butanol–ethanol production from corncob with a new pretreatment technology—wet disk milling. Bioenerg Res. 2013;6(1):35–43.
Karmee SK, Lin CSK. Valorisation of food waste to biofuel: current trends and technological challenges. Sustain Chem Process. 2014;2(1):22.
Carroll A, Somerville C. Cellulosic biofuels. Annu Rev Plant Biol. 2009;60:165–82.
Balat M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manage. 2011;52(2):858–75.
Shaw AJ, Covalla SF, Miller BB, Firliet BT, Hogsett DA, Herring CD. Urease expression in a Thermoanaerobacterium saccharolyticum ethanologen allows high titer ethanol production. Metab Eng. 2012;14(5):528–32.
Hon S, Olson DG, Holwerda EK, Lanahan AA, Murphy JL, Maloney MI, Zheng TY, Papanek B, Guss AM, Lynd LR. The ethanol pathway from Thermoanaerobacterium saccharolyticum improves ethanol production in Clostridium thermocellum. Metab Eng. 2017;42:175–84.
Dürre P, Richard T. Microbial energy conversion revisited. Curr Opin Biotechnol. 2011;22(3):309–11.
Jiang YJ, Guo D, Lu JS, Dürre P, Dong WL, Yan W, Zhang WM, Ma JF, Jiang M, **n FX. Consolidated bioprocessing of butanol production from xylan by a thermophilic and butanologenic Thermoanaerobacterium sp. M5. Biotechnol Biofuels. 2018;11(1):89.
Saggi SK, Dey P. An overview of simultaneous saccharification and fermentation of starchy and lignocellulosic biomass for bio-ethanol production. Biofuels. 2016. https://doi.org/10.1080/17597269.2016.1193837.
Shin HD, Mcclendon S, Vo T, Chen RR. Escherichia coli binary culture engineered for direct fermentation of hemicellulose to a biofuel. Appl Environ Microbiol. 2010;76(24):8150–9.
Ho CY, Chang JJ, Lee SC, Chin TY, Shih MC, Li WH, Huang CC. Development of cellulosic ethanol production process via, co-culturing of artificial cellulosomal Bacillus and kefir yeast. Appl Energy. 2012;100:27–32.
Demain AL, Newcomb M, Wu JHD. Cellulase, clostridia, and ethanol. Microbiol Mol Biol Rev. 2005;69:124–54.
Beguin P, Aubert JP. The biological degradation of cellulose. FEMS Microbiol Rev. 1994;13:25–58.
Lynd LR. Production of ethanol from lignocellulosic materials using thermophilic bacteria: critical evaluation of potential and review. Adv Biochem Eng Biotechnol. 1989;38:1–52.
He Q, Hemme CL, Jiang H, He Z, Zhou J. Mechanisms of enhanced cellulosic bioethanol fermentation by co-cultivation of Clostridium and Thermoanaerobacter spp. Bioresour Technol. 2011;102(20):9586–92.
Zuroff TR, **ques SB, Curtis WR. Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture. Biotechnol Biofuels. 2013;6:59.
** C, Yao M, Liu H, Lee CFF, Ji J. Progress in the production and application of n-butanol as a biofuel. Renew Sust Energ Rev. 2011;15(8):4080–106.
Zheng T, Olson DG, Tian L, Bomble YJ, Himmel ME, Lo J, Hon S, Shaw AJ, van Dijken JP, Lynd LR. Cofactor specificity of the bifunctional alcohol and aldehyde dehydrogenase (AdhE) in wild-type and mutant Clostridium thermocellum and Thermoanaerobacterium saccharolyticum. J Bacteriol. 2015;197(15):2610–9.
Trindade WRDS, Santos RGD. Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew Sust Energ Rev. 2017;69:642–51.
Nguyen S, Ala F, Cardwell C, Cai D, McKindles KM, Lotvola A, Hodges S, Deng Y, Tiquia-Arashiro SM. Isolation and screening of carboxydotrophs isolated from composts and their potential for butanol synthesis. Environ Technol. 2013;34:1995–2007.
Sun C, Zhang S, **n FX, Shanmugam S, Wu YR. Genomic comparison of Clostridium species with the potential of utilizing red algal biomass for biobutanol production. Biotechnol Biofuels. 2018;11:42.
Shanmugam S, Sun C, Zeng X, Wu YR. High-efficient production of biobutanol by a novel Clostridium sp. strain WST with uncontrolled pH strategy. Bioresour Technol. 2018;256:543–7.
Jiang YJ, Chen TP, Dong WL, Zhang M, Zhang WM, Wu H, Ma JF, Jiang M, **n FX. The draft genome sequence of Clostridium beijerinckii NJP7, a unique bacterium capable of producing isopropanol-butanol from hemicellulose through consolidated bioprocessing. Curr Microbiol. 2018;75(3):305–8.
Wen ZQ, Wu MB, Lin YJ, Yang LR, Lin JP, Cen PL. A novel strategy for sequential co-culture of Clostridium thermocellum and Clostridium beijerinckii, to produce solvents from alkali extracted corn cobs. Process Biochem. 2014;49(11):1941–9.
Pfromm PH, Amanor-Boadu V, Nelson R, Vadlani P, Madl R. Bio-butanol vs. bio-ethanol: a technical and economic assessment for corn and switchgrass fermented by yeast or Clostridium acetobutylicum. Biomass Bioenerg. 2010;34(4):515–24.
Saini M, Chen MH, Chiang CJ, Chao YP. Potential production platform of n-butanol in Escherichia coli. Metab Eng. 2015;27:76–82.
Lai EPC. Biodiesel: environmental friendly alternative to petrodiesel. J Pet Environ. Biotechnol. 2014;5:122.
Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14(1):217–32.
Singh A, Olsen SI. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl. Energ. 2011;88(10):3548–55.
Sharma KK, Schuhmann H, Schenk PM. High lipid induction in microalgae for biodiesel production. Energies. 2012;5:1532–53.
Li Y, Han D, Sommerfeld M, Hu Q. Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol. 2011;102(1):123–9.
Dash A, Banerjee R. Enhanced biodiesel production through phyco-myco co-cultivation of Chlorella minutissima and Aspergillus awamori: an integrated approach. Bioresour Technol. 2017;238:502–9.
Yen HW, Chen PW, Chen LJ. The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour Technol. 2015;184:148–52.
Abdel-Rahman MA, Tashiro Y, Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv. 2013;31(6):877–902.
Shahab RL, Luterbacher JS, Brethauer S, Studer MH. Consolidated bioprocessing of lignocellulosic biomass to lactic acid by a synthetic fungal-bacterial consortium. Biotechnol Bioeng. 2018;115:1207–15.
Eiteman MA, Lee SA, Altman R, Altman E. A substrate-selective co-fermentation strategy with Escherichia coli produces lactate by simultaneously consuming xylose and glucose. Biotechnol Bioeng. 2009;102:822–7.
Zhang HR, Li ZJ, Pereira B, Stephanopoulos G. Engineering E. coli–E. coli, co-cultures for production of muconic acid from glycerol. Microb Cell Fact. 2015;14:134.
Bremus C, Herrmann U, Bringer-Meyer S, Sahm H. The use of microorganisms in l-ascorbic acid production. J Biotechnol. 2006;124:196–205.
Zhu Y, Liu J, Liu J, Du G, Zhou J, Chen J. A high throughput method to screen companion bacterium for 2-keto-l-gulonic acid biosynthesis by co-culturing Ketogulonicigenium vulgare. Process Biochem. 2012;47(9):1428–32.
Zhang J, Liu J, Shi Z, Liu L, Chen J. Manipulation of B. megaterium growth for efficient 2-KLG production by K. vulgare. Process Biochem. 2010;45(4):602–6.
Wang EX, Ding MZ, Ma Q, Dong XT, Yuan YJ. Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb Cell Fact. 2016;15:21.
Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–95.
Hainsworth JD, Thompson DS, Greco FA. Paclitaxel by 1-hour infusion: an active drug in metastatic non-small-cell lung cancer. J Clin Oncol. 1995;13(7):1609–14.
Suffness M, Wall ME. Discovery and development of Taxol. In: Suffness M, editor. Taxol science and applications. Boca Raton: CRC Press; 1995. p. 3–25.
Kingston DGI. The shape of the things to come: structural and synthetic studies of Taxol and related compounds. Phytochemistry. 2007;68(14):1844–54.
Engels B, Dahm P, Jennewein S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards taxol (paclitaxel) production. Metab Eng. 2008;10:201–6.
Peng Z, Zhou PP, Yu LJ. An endophytic taxol-producing fungus from taxus media, Cladosporium cladosporioides MD2. Curr Microbiol. 2009;59:227–32.
Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–51.
Chemler JA, Lock LT, Koffas MAG, Tzanakakis ES. Standardized bio-synthesis of flavan-3-ols with effects on pancreatic beta-cell insulin secretion. Appl Microbiol Biotechnol. 2007;77:797–807.
Ola ARB, Thomy D, Lai D, Brötzoesterhelt H, Proksch P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through co-culture with Bacillus subtilis. J Nat Prod. 2013;76(11):2094–9.
Zhuravleva OI, Kirichuk NN, Denisenko VA, Dmitrenok PS, Yurchenko EA, Min′Ko EM, Ivanets EV, Sh Afiyatullov Sh. New diorcinol J produced by co-cultivation of marine fungi Aspergillus sulphureus and Isaria feline. Chem Nat Compd. 2016;52:227–30.
Jagmann N, Philipp B. Design of synthetic microbial communities for biotechnological production processes. J Biotechnol. 2014;184:209–18.
Song H, Ding MZ, Jia XQ, Ma Q, Yuan YJ. Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev. 2014;43(20):6954–81.
Wintermute EH, Silver PA. Emergent cooperation in microbial metabolism. Mol Syst Biol. 2010;6(1):407.
Shong J, Diaz MRJ, Collins CH. Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotechnol. 2012;23(5):798–802.
Schertzer JW, Boulette ML, Whiteley M. More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol. 2009;17(5):189–95.
Choudhary S, Schmidt-Dannert C. Applications of quorum sensing in biotechnology. Appl Microbiol Biotechnol. 2010;86:1267–79.
Pande S, Shitut S, Freund L, Westermann M, Bertels F, Colesie C, Bischofs IB, Kost C. Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat Commun. 2015;6:6238.
Fazzini RAB, Preto MJ, Quintas ACP, Bielecka A, Timmis KN, Dos Santos VAPM. Consortia modulation of the stress response: proteomic analysis of single strain versus mixed culture. Environ Microbiol. 2010;12(9):2436–49.
Santos CN, **ao W, Stephanopoulos G. Rational, combinatorial, and genomic approaches for engineering l-tyrosine production in Escherichia coli. Proc Natl Acad Sci USA. 2012;109(34):13538–43.
**e ZX, Li BZ, Mitchell LA, Wu Y, Qi X, ** Z, et al. “perfect” designer chromosome v and behavior of a ring derivative. Science. 2017;355(6329):eaaf4704.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31700092, 21706125, 21727818, 21706124), the Jiangsu Province Natural Science Foundation for Youths (BK20170993, BK20170997), the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (XTE1834), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX18_1109), the China Postdoctoral Innovative Talents Support Program (BX20180140), Open foundation of Jiangsu Key Laboratory for Biomass-Based Energy and Enzyme Technology (BEETKB1801) and Huaian Science Foundation HAB201702.
Author information
Authors and Affiliations
Contributions
JYJ, WRF, ZJ, HAY and JM conceptualized and organized this review, XJX, ZWM and MJF made the figures and tables. JYJ, WRF and DWL drafted the review. XFX and JM revised the review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jiang, Y., Wu, R., Zhou, J. et al. Recent advances of biofuels and biochemicals production from sustainable resources using co-cultivation systems. Biotechnol Biofuels 12, 155 (2019). https://doi.org/10.1186/s13068-019-1495-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-019-1495-7