Abstract
This study investigated improvements in sugarcane ethanol production by adapting yeast strains for very high gravity fermentation. Two yeast strains (C22 and Y904) were adapted in eight fermentation cycles with increasing initial sugar content from 56.2 to 296.1 g L−1 (Experiment 1). After the last cycle, the “adapted” yeasts were recycled in a wort containing 296.1 g L−1 initial sugar and compared with their respective strains that were not subjected to the adaptation process (Experiment 2). Fermentative parameters were analyzed and the osmotic stress on yeast cell morphology was assessed by scanning electron microscopy (SEM). In Experiment 1, along the fermentation cycles, strain Y904 showed a decrease in cell viability after sugar concentration of 223 g L−1. SEM images showed that Y904 cells were wrinkled after this cycle. In the case of strain C22, no differences in cell viability were observed along the cycles. However, for both strains, the residual sugars were relatively high and the ethanol content was below the maximum potential. In Experiment 2, for strain Y904, no differences were observed between adapted and non-adapted yeasts in terms of ethanol content, cell viability, and morphology. In the case of strain C22, cell viability and final ethanol content were significantly higher in the adapted yeast, which had cells less damaged by the osmotic stress. In conclusion, the study supports the importance of yeast strain selection and adaptation for efficient VHG fermentation, by demonstrating the superior performance of yeast strain C22 in response to increasing initial sugar content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sugarcane is considered a sustainable feedstock for ethanol production [1,2,3]. Sugarcane ethanol has an energy balance of 9.4 renewable energy units produced per unit of fossil fuel used, which is four and seven times higher than ethanol produced from sugar beet and corn, respectively [4]. Moreover, ethanol production in Brazil is expected to increase and become more profitable in the coming years due to RenovaBio. This is a federal government program that allows biofuel producers to receive financial credits (CBIOs) for each ton of CO2 equivalent no longer emitted [5, 6].
However, the sugarcane ethanol production process can be improved and become more sustainable. Vinasse is a dark brown liquid considered the main byproduct of this process as it is produced at a rate of 10 to 15 L per liter of ethanol produced [7]. The volume of vinasse and its concentration depend on the ethanol content at the end of fermentation. For example, if the ethanol content is 7.5% (vv−1), approximately 7.5 L of ethanol and about 92.5 L of vinasse are produced per 100 L of wine. If the ethanol content is increased to 15%, approximately 15 L of ethanol and 85 L of vinasse can be produced per 100 L of wine. Therefore, by increasing the ethanol content, the vinasse generated would decrease from 12.3 to 5.7 L of vinasse generated per liter of ethanol produced.
In the context of RenovaBio, vinasse is emerging as a promising feedstock for biogas production to replace biofuels in field operations and increase the energy balance of sugarcane ethanol [8, 9]. Anaerobic digestion of vinasse can increase the bioenergy production of sugarcane mills by almost 16% [10]. In addition to that, if all the vinasse produced in the State of São Paulo was used for biogas production, the emission of 3.9 million tons of CO2eq per year would be avoided, which could mean an increase in revenue of $78 to $156 million per year due to the commercialization of CBIOs [10].
Fermentation aimed at higher ethanol content also produces a more concentrated vinasse, which can increase the methane yield from anaerobic digestion, reduce reactor volumes, and reduce the amount of vinasse that needs to be handled and applied to the field as fertilizer [10]. The higher ethanol content can also provide other benefits such as increased productivity and reduced water and energy requirements in industrial processes [11]. Furthermore, the distillation process is one of the more energy-intensive processes in the industrial plant, so the higher ethanol concentration will also reduce the energy demand of this specific step [11, 12].
Concentrations of dissolved solids, including fermentable sugars, greatly influence fermentation and other processes in the sugarcane mill. Normal gravity (NG, 15–16 °Brix) ethanol production is a well-established process. After a fermentation with satisfactory efficiency, the wine will have 7–8% ethanol content (vv−1) [13]. Improvements in yeast bioengineering and genetics and new technologies have allowed the concentration of wort to a high gravity (HG, 18 to 24 °Brix) process, increasing the ethanol content to 10 to 12% at the end of fermentation [14]. The very high gravity (VHG) technology is based on the fermentation of wort with more than 27% dissolved solids [15, 16] and can reach final ethanol levels of more than 15%. However, this process still faces challenges for its implementation on an industrial scale.
The high initial sugar content causes osmotic stress to the yeast, significantly reducing cell viability and fermentation efficiency [17,18,19,20]. In addition to that, high ethanol levels can also have negative effects on yeast physiology and reduce cell viability [21]. As a result, high ethanol levels can lead to incomplete fermentation with high residual sugar [16, 22]. Therefore, to achieve a successful implementation of very high gravity technology, it is necessary to select and develop yeast strains with high fermentation efficiency [11]. These strains should be able to tolerate multiple stresses such as high sugar contents at the beginning of fermentation and high ethanol levels at the end.
On the positive side, yeast, as a unicellular organism, has rapid reproduction rates, high adaptability to different environmental conditions, and can be easily multiplied on a small scale. These characteristics make yeast well suited for laboratory-scale selection based on tolerance to specific conditions, paving the way for its application on an industrial scale [23,24,25]. Thus, laboratory-scale fermentations with increasing initial sugar content can be a valuable tool for rapid selection of yeast strains tolerant to high ethanol levels and osmotic stress resulting from high fermentable sugar concentrations [22]. To this end, it is crucial to investigate how different yeast strains respond to increasing sugar levels in the wort and how this affects other fermentative parameters in addition to the final ethanol content.
Based on this background, the aim of this study was to evaluate how the adaptation process of different yeast strains on a laboratory scale affects their fermentation ability at very high gravity. This study consisted of two experiments under sterile conditions. In Experiment 1, eight fermentation cycles with increasing initial sugar content (from 56.2 to 296.1 g L−1) were performed with two yeast strains (Y904 and C22). At the end of the last cycle, the yeasts were collected and designated as “adapted” yeasts. In Experiment 2, the adapted yeast strains were compared with their respective non-adapted strains by evaluating different fermentative parameters.
The hypotheses tested in this study were as follows:
-
H1. The consistent increase in initial sugars after each cycle will affect yeast morphology and fermentation efficiency after each cycle as it can cause an osmotic stress in the yeast strains and will differ between the two strains.
-
H2. The adaptation will improve the yeast’s tolerance to high initial sugar levels at the start of fermentation and high ethanol levels at the end of fermentation compared to their respective non-adapted strains.
2 Materials and methods
The study consisted of two experiments. In the first experiment, two yeast strains were used in fermentations with a constant increase in sugar content for eight cycles, ranging from 56.2 g L−1 (6 °Brix) in the first cycle to 296.1 g L−1 (30.2 °Brix) in the last cycle. In the second experiment, the adapted yeasts were selected and compared with their non-adapted forms in a fermentation kinetics using the same wort as in the last cycle. Figure 1 shows an overview of the experimental design of both experiments. Table 1 shows the wort sugar composition for each cycle of Experiment 1 and Experiment 2.
2.1 Yeasts and wort preparation
In this study, two different yeast (Saccharomyces cerevisiae) strains were assessed. The strain Y904 is a commercial yeast strain in Brazil, from AB Brasil (Pederneiras, Sao Paulo). More information about the fermentation efficiency and suggested conditions of this strain can be found in Alcarde et al. [26] and Cruz et al. [27], respectively. The strain C22 MYCOFERM is from the company Ever Intec, and is usually used for wine fermentations with relatively higher ethanol contents (~13.5%). More information about this strain can be found in Dekker et al. [22] and Douradinho et al. [28].
The wort used in both experiments was obtained by diluting sugarcane syrup with distilled water. The syrup (Brix > 60°) was collected from the Granelli sugarcane mill, located in the municipality of Charqueada, State of São Paulo, Brazil. The syrup was first diluted to 35 °Brix. It was then clarified by filtering and using 2.5 g of monobasic sodium phosphate per liter. The clarified diluted syrup was then autoclaved at 120 °C for 25 min at 1 atm pressure. A detailed description of the clarification and sterilization process can be found in [20]. All the equipment, tools, and flasks used in this study were sterilized each time before use to avoid contamination of the fermentation process.
After these processes, the wort was diluted to achieve the desired initial sugar levels. It is worth noting that prior to the start of the fermentation, a subsample of the wort was taken and analyzed for Brix and sugar levels. These are the values presented in Table 1.
2.2 Experiment 1
In this experiment, the yeast strains were reactivated in the first cycles, as these started with the fermentations with low initial sugar levels. The fermentations were carried out in 500-mL Erlenmeyer flasks with a working volume of 300 mL, fed with a simple batch. The yeasts were inoculated in the first cycle with 3 g of yeast per 100 mL of wort (9 g in total). The flasks were sealed to prevent external contamination. The external temperature was controlled at 28 °C and the flasks were shaken at 80 rpm during the fermentation process. Each treatment had five replicates. The fermentation time of each cycle was determined based on previous studies by Douradinho et al. [22].
The procedure described in Fig. 1 was followed for each cycle. Briefly, the fermented wort was transferred to sterilized containers and centrifuged at 2917g for 10 min. At the end of the fermentation, the wet solid biomass (hereafter referred to as “yeast cream”) was analyzed for final cell viability and inoculated into the wort of the next cycle. The supernatant was analyzed for ethanol, organic acids, and carbohydrates, including residual sugars. More information about the analyses is presented in Sect. 2.4. In the last cycle, the yeast cream was collected and used for Experiment 2. In cycles 1, 2, 5, and 7, the yeast cells were imaged using the scanning electron microscope (SEM).
2.3 Experiment 2
The wort used in this experiment had 296.1 g of fermentable sugar per liter, the same as in the 8th cycle of Experiment 1. Although the yeast strain may have adapted to high initial sugar levels in previous cycles, the “adapted” yeast was selected after this cycle because to be considered a very high gravity fermentation, the initial soluble solids must be at least 270 g L−1, and the experiment was designed to reach these levels in this cycle. For very high gravity, the wort may not provide enough nutrients for the yeast may lead to deficiencies, affecting the fermentation efficiency. For this reason, in these studies, the wort is often supplemented with nutrients. However, the objective of this study was to compare the response of two strains and the addition of other variables, as wort supplementation, was not considered, although it will be relevant for further studies.
In this experiment, both strains were compared in their adapted and non-adapted forms. For the non-adapted forms, for each experimental unit, 9 g of yeast was rehydrated by mixing with distilled water and shaken for 2 h. The solution was then centrifuged and the yeasts were inoculated into their respective reactors. For the adapted yeasts, the yeast creams collected from Experiment 1 were merged with their respective strains. The cell concentration was determined and a subsample was collected and applied to each reactor in Experiment 2, aiming for similar cell concentration in all treatments. The flasks were sealed to prevent external contamination. The external temperature was controlled at 28 °C and the flasks were shaken at 80 rpm during the fermentation process. Each treatment had five replicates. The fermentation lasted 17 h.
The fermented wort was transferred to sterilized containers and centrifuged at 2917g for 10 min. After centrifugation, the yeast cream was analyzed for final cell viability and a sample was collected and imaged with SEM. The supernatant was analyzed for ethanol, organic acids, and carbohydrates, including residual sugars. More information on the analyses is given in Sect. 2.4. The initial pH of the wort used in this experiment was 4.3. However, we did not analyze the pH during and after fermentation. Moreover, the wort was not supplemented with macro- and micronutrients.
2.4 Analyses
2.4.1 Yeast cells
Yeast was analyzed for cell viability at the beginning of Experiments 1 and 2 and at the end of each fermentation in both experiments. Yeast viability was determined by differential staining of live cells with 0.1% methylene blue solution. The stained solution was placed in a Neubauer chamber and dead and live cells were counted by light microscopy according to the method described by Pierce [29] and Oliveira et al. [30]. A more detailed description of this procedure can be found in Sica et al. [20].
In Experiment 1 (end of cycles 1, 2, 5, and 7) and after the fermentation of Experiment 2, the yeast cells from the yeast cream were imaged with a scanning electron microscope (SEM, LEO, model 435 VP). The yeast cream was stored at 4 °C in 0.5 mL Eppendorf’s with Karnovisky’s fixative. The samples were placed on microscope blades with a drop of poly-L-lysine for 20 min. Excess poly-L-lysine was removed with ethanol. The samples were dehydrated with a critical point dehydrator (LEICA, model EM CPD300). The blades were subjected to a metallization process (Bal-Tec, model SCD-050). The scanning electron microscope images were taken at a magnification of 25,000 times.
2.4.2 Ethanol content
For this analysis, 25 mL of the centrifuged supernatant was collected from each experimental unit. These samples were distilled in a micro distiller MA 012/1 (Marconi, Piracicaba, Sao Paulo, Brazil). Distillation was continued until the 25 mL volume was collected in a volumetric flask placed on the outlet valve of the distiller condenser. After distillation, the sample was analyzed and had the ethanol content determined using the Schmidt Haensch Digital Densimeter EDM 400. A more detailed description of this procedure can be found in Sica et al. [20].
2.4.3 Ion chromatography
The supernatant of the centrifuged wine was analyzed for sucrose (not detected), fructose, glucose (residual sugars), glycerol, and mannitol content using a 930 Compact IC Flex ion chromatograph (Metrohm) equipped with an amperometric detector and a Metrospec Carb 1 column. A 100 mmol L−1 NaOH solution was used as the eluent at a flow rate of 1 mL min−1 and the temperature was maintained at 35 °C.
The contents of acetic, lactic, succinic, formic, and aconitic acids in the centrifuged wine were determined by ion chromatography, using the same equipment as previously described (Methrom, model 930 Compact IC Flex), with a conductivity detector, chemical suppressor, and Metrosep Organic 250/7.8 column. The eluent was a solution of 0.5 mM sulfuric acid and 15% acetone, at a flow rate of 1 mL min−1 and the temperature of 25 °C.
2.5 Statistical analyses
All experiments were conducted with five replicates that were randomly distributed within the shaker. Statistical analyses were performed using IBM SPSS Statistics 27.0 software, and graphs were generated using SigmaPlot version 15.0. Levene’s test was used to verify the homogeneity of variance of the data, and the Kolmogrov-Smirnov test was used to verify that the data followed a normal distribution.
In Experiment 1, one-way ANOVA was conducted to assess the effects of cycles (initial sugar contents) on the assessed parameters. In Experiment 2, two-way ANOVA was performed to assess the effects of “strain” and “adaptation” on the assessed parameters. For both experiments, Tukey’s HSD test (< 0.05) was used to compare means. Principal component analysis was performed using SigmaPlot 15.0 only for the results obtained in Experiment 2. To normalize the data, the respective mean was subtracted from each data point and then divided by the standard deviation.
3 Results
3.1 Experiment 1
3.1.1 Residual sugars and cell viability
The cell viability of strain Y904 decreased significantly in cycles 5 and 8, while for strain C22 the only significant difference was observed between cycles 2 and 7, with cycle 8 not significantly different from any of the other cycles (Table 2).
The residual sugars of the fermentations with both yeast strains increased significantly over time, ranging from about 0.3% of the initial sugars in cycle 1 to more than 40% of the initial sugars in cycle 8 (Table 2).
3.1.2 Ethanol content
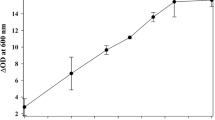
The ethanol content at the end of the fermentation consistently increased until the fifth cycle for both strains. In cycles 6, 7, and 8 the ethanol content had small variations, remaining within the standard error ranges (Fig. 2).
3.1.3 Secondary compounds
The contents of glycerol, mannitol, lactic acid, and acetic acid tended to increase after each cycle. The first one had its higher values in cycle 7, while for the last three, the highest values were observed in cycle 8. In contrast, the succinic acid content of Y904 increased significantly until cycle 6 and was significantly reduced in cycles 7 and 8. For C22, succinic acid increased up to cycle 7 and was significantly reduced in cycle 8 (Table 3).
3.1.4 Yeast cell imaging
In Fig. 3, the SEM image shows that the morphology of the two yeast strains was different as the initial sugar content increased. In the first cycle, the cells of both strains had a round but rough shape. The contrast in the morphology of the strains can be observed throughout the cycles as C22 became more turgid, including cycle 7. For strain Y904, swelling occurred until cycle 5. In cycle 7, the yeasts of strain C22 showed an elongated shape and a wilted appearance.
3.2 Experiment 2
3.2.1 Principal component analysis
In the Principal Component Analysis (PCA) performed on the results obtained in Experiment 2, Component 1 (PC1) corresponded to 55.92% of the variability, while Component 2 (PC2) corresponded to 35.93% of the total variability. The position of the treatments on the graph clearly showed the differences between the strains and the effects of adaptation. Yield and lactic acid and final viability and ethanol content were closely related and close to the C22 adapted. In contrast, aconitic acid, succinic acid, acetic acid, and residual fructose were in an inverted position close to the non-adapted strains (Fig. 4).
3.2.2 Ethanol content and cell viability
The initial total fermentable sugar content of 296.1 g L−1 could provide a theoretical maximum ethanol content of 19.2% at the end of the fermentation. Strain C22, when previously adapted to high initial sugar levels, had the significantly highest ethanol content (14.6% ± 0.4) and final cell viability (83.6% ± 2.7). The non-adapted strain C22 had a significantly higher ethanol content than the non-adapted strain Y904 (13.8% ± 0.2 vs. 13.2% ± 0.2). The non-adapted C22 strain also had a significantly higher final cell viability (68.8% ± 2.0) than the Y904 strain, both adapted (56.7% ± 0.9) and non-adapted (55.2% ± 2.3) (Fig. 5).
Ethanol content (top) and yeast cell viability (bottom) at the end of fermentation for the two yeast strains used in this study (Y904 and C22), previously adapted and non-adapted for Experiment 2, with an initial sugar content of 296 g L−1. The solid line in the top graph indicates the theoretical maximum ethanol content based on stoichiometry (19.2%), considering that the yeast can produce a maximum of 64.75 ml of ethanol per 100 g of fermentable sugars. Different letters indicate a significant difference between treatment (Tukey HSD, < 0.05)
3.2.3 Residual sugars and secondary compounds
The residual sugars ranged from 21.7 to 32.3% of the initial fermentable sugars for the C22 adapted and Y904 non-adapted strains, respectively. The fructose content was significantly higher in both non-adapted strains compared to the previously adapted yeasts (Table 4).
A significant difference was observed for the mannitol content, with the non-adapted Y904 being significantly higher than the adapted C22. Regarding the glycerol content, Y904 had the significantly highest content for both adapted and non-adapted. For strain C22, adapted was significantly lower than non-adapted. Three organic acids showed similar trends, as the contents of succinic, aconitic, and acetic acids were significantly higher in the non-adapted strains compared to the adapted strains. In contrast, lactic acid content was the highest in both adapted strains compared to Y904 non-adapted strains (Table 4).
3.2.4 Yeast cell imaging
In Fig. 6, the SEM image shows that the morphology of the yeast cells at the end of fermentation was affected by the strain and the adaptation process. The cells of strain C22 had a round shape, while the shape of the adapted cells was smooth with a visible bud and the non-adapted cells were rough. For the Y904 strain, both adapted and non-adapted cells were rough, with the non-adapted cells having a more rounded shape and the adapted cells being deformed and wilted (Fig. 6).
4 Discussion
4.1 Yeast strain response to increasing sugar contents (H1)
The first hypothesis tested in this study was that the consistent increase in initial sugars after each cycle will affect yeast morphology and fermentation efficiency after each cycle as it can cause an osmotic stress in the yeast strains and will differ between the two strains.
Douradinho et al. [22] assessed how different feeding systems affected the adaptation of the strain C22 to increasing initial sugar content. They found that fed-batch and simple-batch (the same used in this study) had little effect on fermentation efficiency and final ethanol content [22]. In contrast to it, in a study with initial sugars content of 270 g L−1 using the yeast strain C10, Joannis-Cassan et al. [31] found that a fed-batch reactor could reduce the sugar and ethanol inhibition on yeast fermentation efficiency.
However, even with fed-batch reactors, both studies found that the highest ethanol content obtained was lower than 15% [22, 31]. Cruz et al. [32] investigated different factors such as temperature, sugar concentration, and cellular concentration to improve the very high gravity efficiency of sugarcane juice and molasses with the strain Y904. The authors found that under ideal temperature (27 °C) and cellular concentration (15%) with an initial sugar content of 300 g L−1, the highest ethanol content obtained was also below 15% [32]. Thus, similar to this study, the authors in Douradinho et al. [22] and Cruz et al. [32] found relatively low ethanol contents (< 15%) compared to the theoretical maximum ethanol content (~19%) for an initial sugar content of 300 g L−1. In a very high gravity fermentation with initial sugars of 323 g L−1, Gomes et al. [33] assessed the yeast strain PE-2, commonly used in ethanol distilleries in Brazil. They reached a final ethanol content of 14.9% (wv−1), considerably higher than the levels found in this study. However, they used an artificial fermentation media, supplemented with other nutrients, as nitrogen and magnesium [33].
Douradinho et al. [22] results also showed a significant increase in residual sugars, mainly fructose, when the initial sugar was increased to more than 20%. The high residual sugars indicate an incomplete fermentation, probably due to physicochemical conditions in the substrate that inhibit the yeast from continuing the ethanol production [16, 21, 34]. The higher fructose content is due to the fact that the yeast tends to prioritize the glucose utilization during fermentation [35] due to a higher affinity of the yeast glucose transporting [36]. The residual sugar contents obtained in this study are far from the acceptable under industrial scale, which should be around 2 g L−1 and not higher than 5 g L−1 [37]. In this study, these values were obtained only until the 4th and 5th cycles.
In this study, the fermentations were conducted under sterilized conditions and the results showed a consistent increase in glycerol, mannitol, lactic acid, and acetic acid as the initial sugar content increased. The increase in the content of these compounds can be related to the higher substrate availability to the yeast cells. However, it is worth noting that yeast can produce these secondary compounds to increase its tolerance to stressful conditions [38, 39]. High sugar levels cause osmotic stress, which affects the physiology of the yeast cell by closing the Fps1p channel and causing the accumulation of glycerol in the cell to restore turgor pressure [17]. Glycerol synthesis is a critical mechanism of yeast cells for the rapid release of carbohydrates to yeast under conditions such as osmotic stress, along with other stress-responsive compounds such as trehalose, which can mitigate some of the potential negative effects induced by stress [40, 41].
Yeast can also produce organic acids in response to stress conditions and as a strategy to compete for sugars and nutrients against contaminants [42,43,44,45]. However, the values found in this study are in levels considerably low to the levels in which the organic acids content could cause any negative effects on the yeast performance, which starts to be observed at 10 to 40 g L−1 [12, 46, 47]. Succinic acid is also a secondary product of yeast metabolism against contaminants [48]. According to Bertolini et al. [49], this organic acid is one of the most important factors increasing the acidity during fermentation, as only 1.2 g L−1 (similar to the values obtained in this study) of succinic acid can increase the titratable acidity of the substrate. da Silva et al. [50]observed that in sterile wort fermentation, the lactic and acetic acid contents tended to increase over time during a 44 h fermentation. In contrast, the authors found that succinic acid increased during the first 20 h, reaching almost 2 g L−1, and then decreased to less than 1 g L−1 [50]. This may explain the fact that in this study, unlike the other organic acids, succinic acid did not increase as the initial sugars increased, as the succinic acid could be degraded during the fermentation process, reducing its content at the end of the fermentation.
The increase in the concentration of organic acids synthesized by the yeast can lead to a decrease in pH during fermentation. As a result of lower pH, S. cerevisiae can have a 1.4-fold increase in the expression of 36 genes related to cell wall formation, carbohydrate metabolism, redox metabolism, control of the expression of other genes, nuclear pore transport, protection against various stresses, or coding for proteins with different functions [51].
Based on the previous discussion and the results of Experiment 1, it is possible to claim that our first hypothesis is plausible. Although yeast C22 showed a better adaptation to the increasing initial sugar content compared to Y904, after the 5th cycle the residual sugars as well as lactic and acetic acids increased significantly and the final ethanol content remained between 10 and 12%, which is considerably lower than the theoretical maximum potential ethanol content. Therefore, at initial sugar concentrations above 200 g L−1, the osmotic stress and high ethanol contents reduced the fermentation efficiency.
4.2 Yeast adaption for very high gravity fermentation (H2)
The second hypothesis tested in this study was that the adaptation will improve the yeast’s tolerance to high initial sugar levels at the start of fermentation and high ethanol levels at the end of fermentation compared to their respective non-adapted strains.
Saccharomyces cerevisiae have the ability to respond quickly and effectively to environmental changes. This ability allows the cells to optimize their growth rate under favorable conditions and ensure their survival in adverse environments [52]. The adaptation process to high sugar concentrations in the environment involves osmotic stress, which is triggered by a series of steps. The first stage consists of immediate cellular changes resulting directly from the physico-mechanical forces acting in these conditions. Then, in the second stage, primary defense occurs, characterized by the triggering of processes that initiate cellular protection, repair, and recovery. Finally, in the third and final stage, sustained adaptive events occur that allow the restoration of cellular homeostasis in the face of new circumstances [39]. According to Hohmann [53], in a context where yeast is in homeostatic equilibrium, the water activity of the cytosol and its organelles must be lower than that of the surrounding environment in order to maintain a favorable water volume associated with biochemical reactions. Thus, a constant force is maintained that results in the influx of water into the cell along its concentration gradient. This force is counterbalanced by the turgor pressure, which is determined by the limited extensibility of the plasma membrane and, in particular, the cell wall [53]. However, when yeast is exposed to high osmolarity, it triggers a rapid flow of intracellular water, causing loss of turgor and cell shrinkage. Water is transferred from the vacuole to the cytoplasm, hel** to compensate for the osmotic stress [54].
According to Van Wuytswinkel et al. [18], some of the specific responses induced by osmotic stress include changes in the transcription of stress-responsive genes and the intracellular accumulation of glycerol. These responses are mainly controlled by two signal transduction pathways: (1) the HOG-MAP kinase pathway and (2) the general stress response pathway, which is operationally defined [18]. Exposure of yeast to osmotic pressure results in the expression of common stress-responsive genes, including the HSP12 and CTT1 genes, which are characterized by the presence of general stress-responsive elements in their promoters [55, 56]. Our results showed that the commercial suitability of the yeast strain had a great influence on the adaptation process.
The strain C22 (Mycoferm) is a commercial product of the Italian company Ever Intec and is used for wine fermentation, with the initial sugar contents varying between 210 and 230 g L−1 and reaching a final ethanol content of 13.5% [28]. On the other hand, the Y904 is a strain used for sugarcane juice fermentation with initial sugar levels below the 200 g L−1 [26]. The commercial use of each strain indicates the selection processes they went through before becoming a commercial product and can explain their response to the high gravity fermentation [57]. Based on this, it could be expected that C22, which is commonly used for fermentations with higher initial sugar content, would respond better to the adaptation process.
The different response of each strain is visible in the scanning electron microscopy (SEM) images. In Fig. 3, it is possible to see that at cycle 7 with a very high gravity (27.7 °Brix, 271.6 g L−1), the Y904 cells were deformed, elongated, and with clear shrinkage and roughness. Both ethanol and osmotic stress cause shrinkage of the yeast cell wall and crenation of the outer envelope of the cell wall [57]. The high ethanol levels may have a more pronounced effect on cell morphology [57]. In the presence of elevated ethanol concentrations, the structural integrity of the yeast cell membrane is compromised, leading to swelling or distortion of the mitochondria and the formation of a single, substantial vacuole. Consequently, ethanol exerts its influence on the cell membrane, while the resulting mitochondrial degeneration further promotes the accumulation of intracellular reactive oxygen species, which causes cell aging and death [36, 58]. This can explain the fact that for Y904, the final cell viability decreased after the fifth cycle. As can be seen in the PCA plot (Fig. 4), the cell viability and the final ethanol content are closely related.
However, in this study, the ethanol contents for Y904 in cycles 5 and 7 were similar and, although it is possible to see a roughness of the cell walls in the 5th cycle, it is clear that the cell morphology of Y904 was more affected in cycle 7. This may indicate that Y904 was more susceptible to the osmotic stress than to the high ethanol levels, or that Y904 was continuously exposed to high ethanol levels in cycles 5, 6, and 7, which affected the cell morphology. It is worth noting that even at cycle 7, C22 cells, in contrast to Y904, had a round shape with little or no roughness visible and cell viability did not vary significantly among cycles. According to Canetta et al. (59), the closest and most superficial cell wall folds (see Fig. 6, Y904 and C22 non-adapted) are typical features of osmotic stress.
The results of this study in Experiment 2 demonstrated that the gradual adaptation of the yeast with increasing sugar contents will also affect the fermentation efficiency at very high gravity. As can be seen in the PCA plot (Fig. 4), the C22 adapted was placed close to the yield, final viability, and final ethanol content. As previously discussed, the osmotic stress and high ethanol contents can decrease the cell viability and reduce the fermentation efficiency. In the case of C22 adapted, the final cell viability was significantly higher than the non-adapted C22 and both of the Y904. These differences are also visible in Fig. 6, as, according to Canetta et al. [59], the closest and most superficial cell wall folds observed for Y904 adapted and non-adapted and for C22 non-adapted are typical features of cells under osmotic stress.
The previous adaptation or gradual access to higher sugar contents can mitigate the osmotic stress. Sica et al. [20] conducted a fermentation with concentrated energy cane juice (with initial sugar content of 200 g L−1). In another treatment, they used the same yeast and sugar contents but with corn as the substrate with the saccharification process occurring simultaneously to the fermentation; therefore, the glucose was gradually released during the fermentation. The authors found that the final ethanol content, yield, and fermentation efficiency were significantly lower in the first treatment, probably due to an osmotic stress caused by the direct application of yeast to a high sugar content wort with no pre-adaptation.
In addition, the final ethanol content of the C22 adapted was the highest, but still only 14.3%, which is only 74.4% of the maximum theoretical ethanol content calculated based on stoichiometry, 19.2%. In the case of C22 adapted, considering that 21.7% of the initial sugars remained as residual sugars at the end of the fermentation, 231.8 g L−1 (78.3% of the initial) of fermentable sugars was consumed during the fermentation. According to Gay-Lussac’s optimum fermentation yield, 64.75 mL of ethanol should be produced per 100 g of sugars. Considering this, the maximum ethanol content with consumed sugars should be 15.0%. Therefore, the adapted yeast C22 had an efficient conversion of sugars, since it reached a value of 95.2% of the optimal yield. These values are exceptionally high, since in practice in industrial scale, these values reach a maximum of 92% [60]. However, the residual sugars of around 60 g L−1 are not practical and viable in a commercial scale [37].
In contrast to Experiment 1, slight but significant differences in glycerol levels at the end of fermentation were observed in Experiment 2 (Table 4). As previously discussed, glycerol synthesis is a critical mechanism of yeast cells for the rapid release of carbohydrates under conditions such as osmotic stress to prevent dehydration by balancing the intracellular osmolarity with that of the medium [61]. Despite its lower protective capacity, yeast can also produce mannitol, another sugar alcohol, as a mechanism to adapt to osmotic stress [62]. However, our results also indicate slight but significant differences in mannitol contents at the end of the fermentation. Therefore, we speculate that the production of glycerol and mannitol may not be the main mechanisms responsible for the better response of the strain C22 compared to the Y904.
According to Gonzalez et al. [54], Hog1 is the central player in the cell signaling pathway that regulates the response to osmotic stress. Its primary role is to promote the production and retention of glycerol, an osmolyte, in the cell. High glucose tolerance depends on functional mitochondria. Defects in protein targeting to peroxisomes, GID complex function (which regulates gluconeogenesis), or chromatin dynamics result in reduced survival under sorbitol-induced osmotic stress. Conversely, yeast strains with defects in the endomembrane system gain a competitive advantage under hypertonic conditions, indicating the sensitivity of the Golgi-endosome system to hyperosmolarity [54].
Other compounds such as trehalose may contribute to yeast survival under stress conditions [63]. Therefore, this non-reducing disaccharide, which can constitute up to 10% of the yeast cell as a carbohydrate reserve, may be one of the most (if not the most) important compound in protecting yeast against osmotic stress [64]. However, in this study, we did not analyze the trehalose content at the end of fermentation. Therefore, we suggest that in further experiments with similar objectives, the determination of this compound will be crucial for the interpretation of the results.
Based on our results and the previous discussion, our second hypothesis can be considered partially correct. We observed a clear difference in the response of both evaluated strains to increasing initial sugar levels. Indeed, the C22 adapted achieved the highest ethanol content with high initial sugar contents and it was not visible in its cell morphology and walls some of the typical damage of high ethanol contents and osmotic stress. However, the ethanol content achieved was considerably lower than the maximum theoretical value and although the C22 adapted showed a high efficiency in the conversion of the consumed sugars, it still had a relatively high residual sugar, with more than 20% of the initial sugar content. Therefore, our results indicate that the yeast strain and the adaptation process are essential to improve the efficiency of very high gravity fermentation. However, further strategies may be needed to improve this process.
4.3 Practices to improve very high gravity fermentation and future perspectives
Interestingly, in this study, the high residual sugars were observed even with a relatively high cell viability and the cell with a round shape and no shrinkage in the walls, typical damages of cells affected by high ethanol contents. Therefore, the cells did not show to be affected by the high ethanol content, however, were not capable to complete the fermentation of the residual sugars. As previously discussed, the cell viability is important for achieving a high ethanol content. According to Pereira et al. (2011), at an ethanol content above 140 g L−1 (17.7%), the yeast cell viability decreases considerably. These values, however, are much higher than the ones found in this study [65].
In the alcoholic beverage industry, reinoculation during fermentation with a yeast strain that is both alcohol tolerant and an effective fructose fermenter is a common practice to achieve complete sugar consumption [66]. In the corn ethanol industry, yeast reinoculation can also be done. However, this can increase costs. An alternative is to use a continuous multistage process consisting of two parallel trains of four to five fermenters. The first fermentor is used to increase yeast density and the others are optimized for maximum ethanol production [67, 68]. The yeast density plays an important role on improving the efficiency of very high gravity fermentation [27, 32].
The wort composition will also influence the yeast efficiency at very high gravity fermentations [22]. Silva et al. [69] characterized on an ICP-OES, a syrup similar to the one used in this study, and found that it contained 1.6 g K L−1, 0.48 g P L−1, 0.31 g Mg L−1, 0.27 g Na L−1, 0.15 g Ca L−1, 11.4 mg Zn L−1, 11.4 mg Fe L−1, 12.3 mg Cu L−1, 5.3 mg Mn L−1. In this study, these values were diluted differently in each cycle. Another aspect to be considered is that the mineral content of cane syrup can vary depending on the season, weather conditions, industrial processes, and varieties [70]. In any case, the nutrient composition of cane wort is often lower than the minimum required for ideal conditions for very high gravity fermentation [25, 34] and would require supplementation to improve fermentation efficiency. However, the aim of this study was to compare the response of different strains to the adaptation process. Therefore, we suggest that in further studies with adapted C22, supplementation of wort with macro- and micronutrients would be an important tool to increase the very high gravity fermentation efficiency of pre-adapted yeast.
In the corn ethanol industry, the supplementation with nitrogen sources is a common practice to boost yeast performance [68, 71, 72]. Li et al. [72] found that the supplementation with yeast extract was more efficient than urea and ammonium sulfate on increasing the ethanol yield at very high gravity fermentation of corn hydrolysate. Thomas et al. (1993) found that the supplementation with yeast extract could reduce the fermentation time. In their study, with an initial dissolved solid content of 370 g L−1, they reached an ethanol content of around 23%. However, the fermentation time was 230 h and 130 h for the wort not supplement and the wort supplement with yeast extract, respectively. Moreover, they also had residual sugar of 13.5% of the initial sugar contents, indicating that they had an incomplete fermentation [73].
Jones et al. [16] attributed fermentation arrest not only to nitrogen limitation but also to phosphorus limitation. In their study, by supplementing a wort with diammonium phosphate (20 mM), the ethanol content increased to 14.8% and dissolved solid consumption was almost 80%. Indeed, in a fermentation with different substrates, the results of Sica et al. [20] showed that the phosphate content in the wort was one of the main factors influencing the fermentation efficiency, increasing it by 5%. The primary effects of ethanol toxicity are on the yeast cell membrane, specifically the lipid bilayers. Recent studies using lipidomics have helped to elucidate the mechanisms behind alcohol tolerance in yeast. These studies indicate that yeasts with higher tolerance to elevated ethanol concentrations have increased levels of phosphatidylcholine species. These species have demonstrated the ability to stabilize model membrane bilayers in the presence of high ethanol concentrations. In contrast, strains that are unable to complete fermentation at high ethanol concentrations, resulting in elevated residual sugar levels, tend to have high levels of phosphatidylinositol. Thus, the types and levels of organic phosphorus synthesized and allocated in the lipid bilayers of the cell membrane are critical in determining the ability of the cell to tolerate high ethanol levels [21, 22].
Therefore, besides the selection of ideal yeast strains and the pre-adaptation, the supplementation with nutrients, nitrogen, phosphorus, and adaptations in the industrial processes may be needed in order to reach the desired efficiency of very high gravity fermentation.
5 Conclusions
In conclusion, our study supports the plausibility of the first hypothesis, showing that yeast strain C22 has a better adaptation to increasing initial sugar content compared to Y904. However, after the 5th cycle, a significant increase in residual sugars, lactic and acetic acids, coupled with a final ethanol content between 10 and 12%, suggests a reduced fermentation efficiency at initial sugar concentrations above 200 g L−1 due to osmotic stress and high ethanol levels. Regarding the second hypothesis, our results partially support it. While C22 adapted had the highest ethanol content at elevated initial sugar levels and showed minimal signs of typical damage associated with high ethanol content and osmotic stress, the ethanol content achieved was below the theoretical maximum.
Despite the efficiency of C22 adapted in converting consumed sugars, a relatively high residual sugar (more than 20% of the initial sugar content) suggests the need for further improvements. Our results highlight the critical role of yeast strain selection and adaptation in improving the efficiency of very high gravity fermentations. In addition, strategies such as nutrient and nitrogen/phosphorus supplementation as well as industrial process adaptations may be essential to achieve the desired efficiency in this fermentation process.
Data availability
Data will be make available on request to the corresponding author.
References
Goldemberg J, Mello FFC, Cerri CEP et al (2014) Meeting the global demand for biofuels in 2021 through sustainable land use change policy. Energy Policy 69:14–18. https://doi.org/10.1016/j.enpol.2014.02.008
Goldemberg J (2007) Ethanol for a sustainable energy future. Sci AAAS 315:808–810. https://doi.org/10.1126/science.1137013
Goldemberg J (2007) Ethanol for a sustainable energy future. Science (1979) 315:808–810. https://doi.org/10.1126/science.1137013
Altieri A (2012) Bioethanol Development in Brazil. Compr Renew Energy 5:15–26. https://doi.org/10.1016/B978-0-08-087872-0.00504-7
Grassi MCB, Pereira GAG (2019) Energy-cane and RenovaBio: Brazilian vectors to boost the development of Biofuels. Ind Crop Prod 129:201–205. https://doi.org/10.1016/j.indcrop.2018.12.006
ANP (2019) RenovaCalc. In: Brazilian National Agency of Petroleum, Natural Gas, and Biofuels. http://www.anp.gov.br/producao-de-biocombustiveis/renovabio/renovacalc. Accessed 10 Nov 2019
Godoi LAG, Camiloti PR, Bernardes AN et al (2019) Seasonal variation of the organic and inorganic composition of sugarcane vinasse: main implications for its environmental uses. Environ Sci Pollut Res 26:29267–29282. https://doi.org/10.1007/s11356-019-06019-8
Silva Neto JV, Gallo WLR, Nour EAA (2020) Production and use of biogas from vinasse: implications for the energy balance and GHG emissions of sugar cane ethanol in the Brazilian context. Environ Prog Sustain Energy 39:1–11. https://doi.org/10.1002/ep.13226
Silva Neto JV, Gallo WLR (2021) Potential impacts of vinasse biogas replacing fossil oil for power generation, natural gas, and increasing sugarcane energy in Brazil. Renew Sustain Energy Rev 135. https://doi.org/10.1016/j.rser.2020.110281
Sica P, Marabesi AO, Seleghim AR et al (2024) Effects of vinasse concentration on biogas production: an experimental work and case study in the context of RenovaBio in Brazil. Bioresour Technol Rep 25:101698. https://doi.org/10.1016/j.biteb.2023.101698
Gomes D, Cruz M, de Resende MM et al (2021) Very high gravity bioethanol revisited: main challenges and advances. Fermentation 7:38. https://doi.org/10.3390/fermentation7010038
Maiorella BL, Blanch HW, Wilke CR (1984) Economic evaluation of alternative ethanol fermentation processes. Biotechnol Bioeng 26:1003–1025. https://doi.org/10.1002/bit.260260902
Arshad M, Khan ZM, Khalil-ur-Rehman et al (2008) Optimization of process variables for minimization of byproduct formation during fermentation of blackstrap molasses to ethanol at industrial scale. Lett Appl Microbiol 47:410–414. https://doi.org/10.1111/j.1472-765X.2008.02446.x
Arshad M, Hussain T, Iqbal M, Abbas M (2017) Enhanced ethanol production at commercial scale from molasses using high gravity technology by mutant S. Cerevisiae. Brazilian J Microbiol 48:403–409. https://doi.org/10.1016/j.bjm.2017.02.003
Thomas KC, Hynes SH, Ingledew WM (1996) Practical and theoretical considerations in the production of high concentrations of alcohol by fermentation. Process Biochem 31:321–331. https://doi.org/10.1016/0032-9592(95)00073-9
Jones AM, Thomas KC, Ingledew WM (1994) Ethanolic Fermentation of Blackstrap Molasses and SugarCane Juice using very high gravity technology. J Agric Food Chem 42:1242–1246. https://doi.org/10.1021/jf00041a037
Hohmann S (1997) Sha** up: the responses of yeast to osmotic stress. In: Hohmann S, Mager WH (eds) Yeast Stress Responses, 1st ed. R. G. Landes Company, Austin, Texas, pp 101–146
Van Wuytswinkel O, Reiser V, Siderius M et al (2000) Response of Saccharomyces cerevisiae to severe osmotic stress: evidence for a novel activation mechanism of the HOG MAP kinase pathway. Mol Microbiol 37:382–397. https://doi.org/10.1046/j.1365-2958.2000.02002.x
Bai FW, Chen LJ, Zhang Z et al (2004) Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J Biotechnol 110:287–293. https://doi.org/10.1016/j.jbiotec.2004.01.017
Sica P, Prado LMLM, Granja P et al (2021) Effects of Energy Cane (Saccharum spp.) juice on corn ethanol (Zea mays) fermentation efficiency: integration towards a more sustainable production. Fermentation 7:30. https://doi.org/10.3390/FERMENTATION7010030
Henderson CM, Block DE (2014) Examining the role of membrane lipid composition in determining the ethanol tolerance of Saccharomyces cerevisiae. Appl Environ Microbiol 80:2966–2972. https://doi.org/10.1128/AEM.04151-13
Douradinho R, Sica P, Tonoli F et al (2023) Osmotic stress alleviation in Saccharomyces cerevisiae for high ethanol fermentations with different wort substrates. Stresses 3:813–826. https://doi.org/10.3390/stresses3040055
Johnson EA, Echavarri-Erasun C (2011) Yeast biotechnology. In: Kurtzman CP, Fell JW, Boekhout T (eds) Yeast biotechnology, 5th edn. Elsevier B.V., Amsterdam, pp 21–44
Walker GM, Walker RSK (2018) Enhancing yeast alcoholic fermentations. Adv Appl Microbiol 1:87–129. https://doi.org/10.1016/bs.aambs.2018.05.003
Walker G (1998) Yeast metabolism. In: Walker G (ed) Yeast physiology and biotechnology, 1st edn. John Wiley & Sons Ltd., Chichester, England, pp 203–255
Alcarde AR, Monteiro BM, dos Belluco S S (2012) Composição química De aguardentes de cana-de-açúcar fermentadas por diferentes cepas de levedura Saccharomyces cerevisiae. Quim Nova 35:1612–1618. https://doi.org/10.1590/S0100-40422012000800022
Cruz ML, de Resende MM, Ribeiro EJ (2018) Evaluation of process conditions in the performance of yeast on alcoholic fermentation. Chem Eng Commun 205:846–855. https://doi.org/10.1080/00986445.2017.1423061
Dekker S, Fedrizzi B, van Leeuwen KA et al (2022) Polysulfides accumulation in white wines produced from different oenological yeasts. J Food Compos Anal 111:104632. https://doi.org/10.1016/j.jfca.2022.104632
Pierce JS (1970) Analysis committee measurement of yeast viability. J Inst Brewery 76:442–443. https://doi.org/10.1002/j.2050-0416.1970.tb03325.x
Oliveira A, Gallo C, Alcarde V et al (1996) Methods for microbiological control in sugar and alcohol production (in Portuguese). FERMENTEC/ESALQ/FEALQ Piracicaba
Joannis-Cassan C, Riess J, Jolibert F, Taillandier P (2014) Optimization of very high gravity fermentation process for ethanol production from industrial sugar beet syrup. Biomass Bioenergy 70:165–173. https://doi.org/10.1016/j.biombioe.2014.07.027
Cruz ML, de Resende MM, Ribeiro EJ (2021) Improvement of ethanol production in fed-batch fermentation using a mixture of sugarcane juice and molasse under very high-gravity conditions. Bioprocess Biosyst Eng 44:617–625. https://doi.org/10.1007/s00449-020-02462-x
Gomes DG, Guimarães PMR, Pereira FB et al (2012) Plasmid-mediate transfer of FLO1 into industrial Saccharomyces cerevisiae PE-2 strain creates a strain useful for repeat-batch fermentations involving flocculation–sedimentation. Bioresour Technol 108:162–168. https://doi.org/10.1016/j.biortech.2011.12.089
Douradinho R, Sica P, Oliveira M et al (2024) Assessing Ionizing Radiation and Chlorine Dioxide (ClO2) as potential aseptization treatments for yeast recycling on Mixed Wort of Corn and Sugarcane in Brazil. Stresses 4:155–171. https://doi.org/10.3390/stresses4010009
Berthels N, Cordero-Otero R, Bauer F et al (2004) Discrepancy in glucose and fructose utilisation during fermentation by wine yeast strains. FEMS Yeast Res 4:683–689. https://doi.org/10.1016/j.femsyr.2004.02.005
Bisson LF, Fraenkel DG (1983) Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences 80:1730–1734. https://doi.org/10.1073/pnas.80.6.1730
Bai FW, Anderson WA, Moo-Young M (2008) Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv 26:89–105. https://doi.org/10.1016/j.biotechadv.2007.09.002
Lam FH, Ghaderi A, Fink GR, Stephanopoulos G (2014) Engineering alcohol tolerance in yeast. Science (1979) 346:71–75. https://doi.org/10.1126/science.1257859
Mager W, Siderius M (2002) Novel insights into the osmotic stress response of yeast. FEMS Yeast Res 2:251–257. https://doi.org/10.1016/S1567-1356(02)00116-2
Klein M, Swinnen S, Thevelein JM, Nevoigt E (2017) Glycerol metabolism and transport in yeast and fungi: established knowledge and ambiguities. Environ Microbiol 19:878–893. https://doi.org/10.1111/1462-2920.13617
Mattenberger F, Sabater-Muñoz B, Hallsworth JE, Fares MA (2017) Glycerol stress in Saccharomyces cerevisiae: Cellular responses and evolved adaptations. Environ Microbiol 19:990–1007. https://doi.org/10.1111/1462-2920.13603
Giannattasio S, Guaragnella N, Ždralević M, Marra E (2013) Molecular mechanisms of Saccharomyces cerevisiae stress adaptation and programmed cell death in response to acetic acid. Front Microbiol 4:1–7. https://doi.org/10.3389/fmicb.2013.00033
Graves T, Narendranath NV, Dawson K, Power R (2006) Effect of pH and lactic or acetic acid on ethanol productivity by Saccharomyces cerevisiae in corn mash. J Ind Microbiol Biotechnol 33:469–474. https://doi.org/10.1007/s10295-006-0091-6
Graves T, Narendranath NV, Dawson K, Power R (2007) Interaction effects of lactic acid and acetic acid at different temperatures on ethanol production by Saccharomyces cerevisiae in corn mash. Appl Microbiol Biotechnol 73:1190–1196. https://doi.org/10.1007/s00253-006-0573-5
Narendranath NV, Hynes SH, Thomas KC, Ingledew WM (1997) Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl Environ Microbiol 63:4158–4163. https://doi.org/10.1128/aem.63.11.4158-4163.1997
Ngang JJE, Letourneau F, Wolniewicz E, Villa P (1990) Applied Microbiology Biotechnology Inhibition of beet molasses alcoholic fermentation by lactobacilli. Appl Microbiol Biotechnol 33:490–493
Cherubin RA (2003) Efeitos da viabilidade da levedura e da contaminação bacteriana na fermentação alcoólica
Basso LC, Alves DMG, Amorim HV (1997) The antibacterial action of succinic acid formation by yeast during fermentation. Revista De Microbiol 28:77–82
Bertolini L, Zambonelli C, Giudici P, Castellari L (1996) Higher Alcohol production by Cryotolerant Saccharomyces Strains. Am J Enol Viticultureerican J Enol Viticulture 47:343–345
da Silva APM, Sica P, de AN Pires L et al (2023) Integration of corn and cane for ethanol production: effects of Lactobacilli contamination on fermentative parameters and use of ionizing radiation treatment for disinfection. Fermentation 9:89. https://doi.org/10.3390/fermentation9020089
Gibson BR, Lawrence SJ, Leclaire JPR et al (2007) Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol Rev 31:535–569. https://doi.org/10.1111/j.1574-6976.2007.00076.x
Siderius M, Van Wuytswinkel O, Reijenga KA et al (2000) The control of intracellular glycerol in Saccharomyces cerevisiae influences osmotic stress response and resistance to increased temperature. Mol Microbiol 36:1381–1390. https://doi.org/10.1046/j.1365-2958.2000.01955.x
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372. https://doi.org/10.1128/MMBR.66.2.300-372.2002
Gonzalez R, Morales P, Tronchoni J et al (2016) New genes involved in osmotic stress tolerance in Saccharomyces cerevisiae. Front Microbiol 7. https://doi.org/10.3389/fmicb.2016.01545
Saxena A, Sitaraman R (2016) Osmoregulation in Saccharomyces cerevisiae via mechanisms other than the high-osmolarity glycerol pathway. Microbiol (N Y) 162:1511–1526. https://doi.org/10.1099/mic.0.000360
Moskvina E, Schüller C, Maurer CTC et al (1998) A search in the genome of Saccharomyces cerevisiae for genes regulated via stress response elements. Yeast 14:1041–1050 (https://doi.org/10.1002/(SICI)1097-0061(199808)14:11<1041::AID-YEA296>3.0.CO;2-4 )
Pratt PL, Bryce JH, Stewart GG (2003) The effects of osmotic pressure and ethanol on yeast viability and morphology. J Inst Brew 109:218–228. https://doi.org/10.1002/j.2050-0416.2003.tb00162.x
Ma M, Han P, Zhang R, Li H (2013) Ultrastructural changes of Saccharomyces cerevisiae in response to ethanol stress. Can J Microbiol 59:589–597. https://doi.org/10.1139/cjm-2012-0745
Canetta E, Adya AK, Walker GM (2006) Atomic force microscopic study of the effects of ethanol on yeast cell surface morphology. FEMS Microbiol Lett 255:308–315. https://doi.org/10.1111/j.1574-6968.2005.00089.x
Baeyens J, Kang Q, Appels L et al (2015) Challenges and opportunities in improving the production of bio-ethanol. Prog Energy Combust Sci 47:60–88. https://doi.org/10.1016/j.pecs.2014.10.003
Aslankoohi E, Rezaei MN, Vervoort Y et al (2015) Glycerol production by fermenting yeast cells is essential for Optimal Bread Dough Fermentation. PLoS ONE 10:e0119364. https://doi.org/10.1371/journal.pone.0119364
Shen B, Hohmann S, Jensen RG, Bohnert and HJ (1999) Roles of Sugar alcohols in osmotic stress adaptation. Replacement of glycerol by Mannitol and Sorbitol in yeast. Plant Physiol 121:45–52. https://doi.org/10.1104/pp.121.1.45
Hounsa C-G, Brandt EV, Thevelein J et al (1998) Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiol (N Y) 144:671–680. https://doi.org/10.1099/00221287-144-3-671
Eleutherio E, Panek A, De Mesquita JF et al (2015) Revisiting yeast trehalose metabolism. Curr Genet 61:263–274. https://doi.org/10.1007/s00294-014-0450-1
Pereira FB, Guimarães PMR, Teixeira JA, Domingues L (2011) Robust industrial Saccharomyces cerevisiae strains for very high gravity bio-ethanol fermentations. J Biosci Bioeng 112:130–136. https://doi.org/10.1016/j.jbiosc.2011.03.022
Santos J, Sousa MJ, Cardoso H et al (2008) Ethanol tolerance of sugar transport, and the rectification of stuck wine fermentations. Microbiol (N Y) 154:422–430. https://doi.org/10.1099/mic.0.2007/011445-0
Ingledew WM (2003) Continuous fermentation in the fuel alcohol industry: how does the technology affect yeast? In: Jacques KA, Lyons TP, Kelsall DR (eds) The Alcohol Textbook: a reference for beverage, fuel, and industrial alcohol industries, 4th edn. Nottingham University, Nottingham, UK, pp 135–143
Khullar E, Kent AD, Leathers TD et al (2013) Contamination issues in a continuous ethanol production corn wet milling facility. World J Microbiol Biotechnol 29:891–898. https://doi.org/10.1007/s11274-012-1244-6
Silva ND, Alvim MR, Rosario CGA et al (2022) Nutrients’ supplementation impacts on alcholoic fermentation of corn and sugarcane mixed wort. Res Square Prepriting 1–26. https://doi.org/10.21203/rs.3.rs-1761833/v1
de Souza RB, de Menezes JAS, de Souza R, de FR et al (2015) Mineral composition of the Sugarcane Juice and its influence on the ethanol fermentation. Appl Biochem Biotechnol 175:209–222. https://doi.org/10.1007/s12010-014-1258-7
Thomas KC, Hynes SH, Ingledew WM (2001) Effect of lactobacilli on yeast growth, viability and batch and semi-continuous alcoholic fermentation of corn mash. J Appl Microbiol 90:819–828. https://doi.org/10.1046/j.1365-2672.2001.01311.x
Li Z, Wang D, Shi YC (2017) Effects of nitrogen source on ethanol production in very high gravity fermentation of corn starch. J Taiwan Inst Chem Eng 70:229–235. https://doi.org/10.1016/j.jtice.2016.10.055
Thomas KC, Hynes SH, Jones AM, Ingledew WM (1993) Production of fuel alcohol from wheat by VHG technology. Appl Biochem Biotechnol 43:211–226. https://doi.org/10.1007/BF02916454
Funding
Open access funding provided by Copenhagen University This project was funded by the Brazilian agency “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.”
Author information
Authors and Affiliations
Contributions
Sica, P.: methodology, validation, formal analysis, investigation, data curation, writing original draft, review, and editing, visualization.
Tonoli, F.: methodology, validation, formal analysis, investigation, data curation, writing—revision, project administration
Silverio, M. S.; Douradinho, R.: methodology, investigation, data curation, writing—revision
Mota, L. A.; Prado, L.; Leite, G. M. G. L.; Carvalho, R. S.; Pinto, A. U.: methodology, investigation, writing—revision
Baptista, A. S.: validation, formal analysis, investigation, data curation, writing—revision, project administration, resources, funding acquisition
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sica, P., Tonoli, F., Silverio, M.S. et al. Pre-adaptation of yeast (Saccharomyces cerevisiae) strains to very high gravity can improve fermentation parameters and reduce osmotic stress. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05746-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05746-4