Abstract
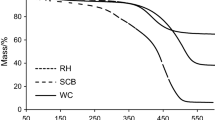
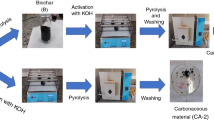
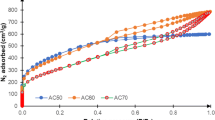
To mitigate the environmental impact of forestry waste, this study presents an innovative approach to convert waste bamboo chips (WB) into high-value activated carbon via pyrolysis, followed by KOH activation of the resulting semi-coke (SC). This process produces activated carbon with an exceptionally high specific surface area, suitable for CO2 adsorption applications. We employed comprehensive thermogravimetric analysis (TG), gas chromatography-mass spectrometry (GC–MS), and Fourier transform infrared spectroscopy (FT-IR) to characterize the pyrolysis behavior and reaction kinetics of WB. The primary mass loss in WB pyrolysis, amounting to 82.8 wt.%, occurs during devolatilization at 270–430 °C. The Coats-Redfern method estimates an average activation energy of 64.2 kJ/mol. The pyrolysis gas predominantly comprises carbon-containing components. Optimal activation with a KOH-to-biochar ratio of 2:1 yielded activated carbon with a surface area of 2883 m2/g. This activated carbon demonstrated a maximum CO2 adsorption capacity of 4.14 mmol/g. The successful application of WB pyrolysis products in bioenergy illustrates its potential for diverse environmental applications.












Similar content being viewed by others
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Cruz OF, Gómez IC, Rodríguez-Reinoso F et al (2023) Activated carbons with high micropore volume obtained from polyurethane foams for enhanced CO2 adsorption[J]. Chem Eng Sci 273:118671
Azevedo VG, Sartori S, Campos LMS (2018) CO2 emissions: a quantitative analysis among the BRICS nations[J]. Renew Sustain Energy Rev 81:107–115
Mohd Azmi NZ, Buthiyappan A, Abdul Raman AA et al (2022) Recent advances in biomass based activated carbon for carbon dioxide capture – a review[J]. J Ind Eng Chem 116:1–20
Kumar M, Gupta A, Thakur IS (2016) Carbon dioxide sequestration by chemolithotrophic oleaginous bacteria for production and optimization of polyhydroxyalkanoate[J]. Biores Technol 213:249–256
Liang Z, Fu K, Idem R et al (2016) Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J]. Chin J Chem Eng 24(2):278–288
Rios RVRA, Martínez-Escandell M, Molina-Sabio M et al (2006) Carbon foam prepared by pyrolysis of olive stones under steam[J]. Carbon 44(8):1448–1454
Ello A S, de Souza L K C, Trokourey A, et al. Development of microporous carbons for CO2 capture by KOH activation of African palm shells[J]. Journal of CO2 Utilization, 2013,2:35–38.
Torrellas SÁ, García Lovera R, Escalona N et al (2015) Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions[J]. Chem Eng J 279:788–798
Labus K, Gryglewicz S, Machnikowski J (2014) Granular KOH-activated carbons from coal-based cokes and their CO2 adsorption capacity[J]. Fuel 118:9–15
Castro MMD, Martínez-Escandell M, Molina-Sabio M et al (2010) Hydrogen adsorption on KOH activated carbons from mesophase pitch containing Si, B, Ti or Fe[J]. Carbon 48(3):636–644
Chunlan L, Shao** X, Yixiong G et al (2005) Effect of pre-carbonization of petroleum cokes on chemical activation process with KOH[J]. Carbon 43(11):2295–2301
Modak A, Bhaumik A (2015) Porous carbon derived via KOH activation of a hypercrosslinked porous organic polymer for efficient CO2, CH4, H2 adsorptions and high CO2/N2 selectivity[J]. J Solid State Chem 232:157–162
Silvestre-Albero A, Silvestre-Albero J, Martinez-Escandell M et al (2015) Novel synthesis of a micro-mesoporous nitrogen-doped nanostructured carbon from polyaniline[J]. Microporous Mesoporous Mater 218:199–205
Soomro A, Chen S, Ma S et al (2020) Promoting effect of ZrO2/CeO2 addition on Fe/CaO catalyst for hydrogen gas production in the gasification process[J]. Biomass Bioenerg 142:105712
Han H, Lou Z, Wang Q, et al (2024) Introducing rich heterojunction surfaces to enhance the high-frequency electromagnetic attenuation response of flexible fiber-based wearable absorbers[J]. Adv Fib Mater 2024. https://doi.org/10.1007/s42765-024-00387-8
Kumar M, **ong X, Wan Z et al (2020) Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials[J]. Biores Technol 312:123613
Ong HC, Chen W, Singh Y et al (2020) A state-of-the-art review on thermochemical conversion of biomass for biofuel production: a TG-FTIR approach[J]. Energy Convers Manage 209:112634
Morya R, Kumar M, Shekhar Thakur I. Bioconversion of syringyl lignin into malic acid by Burkholderia sp. ISTR5[J]. Bioresource Technology, 2021,330:124981.
Hu X, Ding Z, Zimmerman AR et al (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis[J]. Water Res 68:206–216
Dai J, Meng X, Zhang Y et al (2020) Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water[J]. Biores Technol 311:123455
Wang S, Ai S, Nzediegwu C et al (2020) Carboxyl and hydroxyl groups enhance ammonium adsorption capacity of iron (III) chloride and hydrochloric acid modified biochars[J]. Biores Technol 309:123390
Fu Y, Shen Y, Zhang Z et al (2019) Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption[J]. Sci Total Environ 646:1567–1577
Oginni O, Singh K, Oporto G et al (2019) Influence of one-step and two-step KOH activation on activated carbon characteristics[J]. Bioresource Technology Reports 7:100266
Deng L, Zhao Y, Sun S et al (2024) Preparation of corn straw-based carbon by “carbonization-KOH activation” two-step method: gas–solid product characteristics, activation mechanism and hydrogen storage potential[J]. Fuel 358:130134
Yang K, Peng J, Srinivasakannan C et al (2010) Preparation of high surface area activated carbon from coconut shells using microwave heating[J]. Biores Technol 101(15):6163–6169
Neme I, Gonfa G, Masi C (2022) Preparation and characterization of activated carbon from castor seed hull by chemical activation with H3PO4[J]. Results in Materials 15:100304
Donald J, Ohtsuka Y, Xu CC (2011) Effects of activation agents and intrinsic minerals on pore development in activated carbons derived from a Canadian peat[J]. Mater Lett 65(4):744–747
Durán-Jiménez G, Rodriguez J, Stevens L et al (2024) Microwave pyrolysis of waste biomass and synthesis of micro-mesoporous activated carbons: the role of textural properties for CO2 and textile dye adsorption[J]. Chem Eng J 488:150926
Çepelioğullar Ö, Pütün AE (2013) Thermal and kinetic behaviors of biomass and plastic wastes in co-pyrolysis[J]. Energy Convers Manage 75:263–270
Idris SS, Rahman NA, Ismail K et al (2010) Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA)[J]. Biores Technol 101(12):4584–4592
Zhou L, Luo T, Huang Q (2009) Co-pyrolysis characteristics and kinetics of coal and plastic blends[J]. Energy Convers Manage 50(3):705–710
White JE, Catallo WJ, Legendre BL (2011) Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies[J]. J Anal Appl Pyrol 91(1):1–33
Chen R, Zhang S, Cong K et al (2020) Insight into synergistic effects of biomass-polypropylene co-pyrolysis using representative biomass constituents[J]. Biores Technol 307:123243
Masnadi MS, Habibi R, Kopyscinski J et al (2014) Fuel characterization and co-pyrolysis kinetics of biomass and fossil fuels[J]. Fuel 117:1204–1214
Zhou C, Liu G, Wang X et al (2016) Co-combustion of bituminous coal and biomass fuel blends: thermochemical characterization, potential utilization and environmental advantage[J]. Biores Technol 218:418–427
Dong Q, **ong Y (2014) Kinetics study on conventional and microwave pyrolysis of moso bamboo[J]. Biores Technol 171:127–131
Basu P. Chapter 3 - pyrolysis and torrefaction[M]//Basu P. Biomass gasification and pyrolysis. Boston: Academic Press, 2010:65–96.
Neves D, Thunman H, Matos A et al (2011) Characterization and prediction of biomass pyrolysis products[J]. Prog Energy Combust Sci 37(5):611–630
Gautam N, Chaurasia A (2020) Study on kinetics and bio-oil production from rice husk, rice straw, bamboo, sugarcane bagasse and neem bark in a fixed-bed pyrolysis process[J]. Energy 190:116434
Heidari A, Stahl R, Younesi H et al (2014) Effect of process conditions on product yield and composition of fast pyrolysis of Eucalyptus grandis in fluidized bed reactor[J]. J Ind Eng Chem 20(4):2594–2602
Collard F, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin[J]. Renew Sustain Energy Rev 38:594–608
Wang B, Xu F, Zong P et al (2019) Effects of heating rate on fast pyrolysis behavior and product distribution of Jerusalem artichoke stalk by using TG-FTIR and Py-GC/MS[J]. Renewable Energy 132:486–496
Wang T, Hou H, Ye Y et al (2019) Combustion behavior of refuse-derived fuel produced from sewage sludge and rice husk/wood sawdust using thermogravimetric and mass spectrometric analyses[J]. J Clean Prod 222:1–11
Huo Y, Guo Z, Liu Y et al (2021) Addressing unresolved complex mixture of I/SVOCs emitted from incomplete combustion of solid fuels by nontarget analysis[J]. J Geophys Res D. Atmosph: JGR (23):126. https://doi.org/10.1029/2021JD035835
Yu S, Wang L, Li Q et al (2022) Sustainable carbon materials from the pyrolysis of lignocellulosic biomass[J]. Materials Today Sustainability 19:100209
Zhang B, Ren J, Gu X et al (2011) A method for the preparation of activated carbon based carbon/carbonaceous composites with controllable surface functionality[J]. J Porous Mater 18(6):743–750
Zhang X, Sun B, Fan X et al (2021) Building relationships between molecular composition of carbon precursor and capacitance of a hierarchical porous carbon-based supercapacitor[J]. https://doi.org/10.1021/ACSAEM.0C02915
Wu R, Ye Q, Wu K, et al. Highly efficient CO2 adsorption of corn kernel-derived porous carbon with abundant oxygen functional groups[J]. Journal of CO2 Utilization, 2021,51(1):101620.
Zhang S, Zhang X, Zhang S et al (2023) Biomass-derived functional carbon material for CO2 adsorption and electrochemical CO2 reduction reaction[J]. Carbon Capture Science & Technology 9:100135
Reiche S, Blume R, Zhao XC et al (2014) Reactivity of mesoporous carbon against water – an in-situ XPS study[J]. Carbon 77:175–183
Tian X, Yu J, Qiu L et al (2023) Structural changes and electrochemical properties of mesoporous activated carbon derived from Eucommia ulmoides wood tar by KOH activation for supercapacitor applications[J]. Ind Crops Prod 197:116628
Jang E, Choi SW, Hong S et al (2018) Development of a cost-effective CO2 adsorbent from petroleum coke via KOH activation[J]. Appl Surf Sci 429:62–71
Alabadi A, Razzaque S, Yang Y et al (2015) Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity[J]. Chem Eng J 281:606–612
Han J, Zhang L, Zhao B et al (2019) The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption[J]. Ind Crops Prod 128:290–297
Huang G, Liu Y, Wu X et al (2019) Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance[J]. New Carbon Mater 34(3):247–257
Yue L, **a Q, Wang L et al (2018) CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell[J]. J Colloid Interface Sci 511:259–267
Fiuza-Jr RA, Andrade RC, Andrade HMC (2016) CO2 capture on KOH-activated carbons derived from yellow mombin fruit stones[J]. J Environ Chem Eng 4(4, Part A):4229–4236.e 57. https://doi.org/10.1016/j.jece.2016.09.025
Wu W, Wu C, Liu J et al (2024) Nitrogen-doped porous carbon through K2CO3-activated bamboo shoot shell for an efficient CO2 adsorption[J]. Fuel 363:130937
Manyà JJ, González B, Azuara M et al (2018) Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity[J]. Chem Eng J 345:631–639
Funding
Key R&D Special Project of Hubei Provincial Technology Innovation Plan in 2023 (Social Development Field) (Grants No. 2023BCB038 and 111 Project Grant No. B17019).
Author information
Authors and Affiliations
Contributions
The corresponding author is responsible for ensuring that the descriptions are accurate and agreed by all authors, the role(s) of all authors are as follows:
Yonghui Xu: resources, data curation.
Wei Zhan: software calculation.
Yiyun Liu: writing—reviewing and editing.
Dingle Zhang: visualization, investigation, project administration.
Yi Xu: project administration.
Zhengshun Wu: supervision and formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Z., Liu, Y., Xu, Y. et al. Preparation of activated carbon through the pyrolysis of waste bamboo chips and evaluation of its CO2 adsorption efficacy. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05735-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05735-7