Abstract
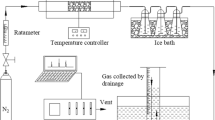
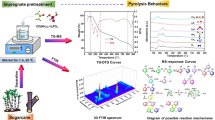
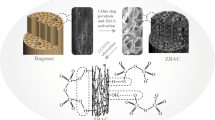
Nowadays, global warming is mainly caused by increase in CO2 concentration in the environment; to mitigate this problem, an attractive strategy for efficient CO2 emission reduction was applied: carbon capture. Therefore, this work had as main objective to measure the capture of CO2, through two carbonaceous materials produced from sugar cane bagasse; this biochar was activated chemically with KOH, by two different processes: the impregnated biochar (CA-1) was taken to pyrolysis process at 350 °C and another sugarcane bagasse sample was also impregnated, but directly in dry biomass (CA-2), obtaining two carbonaceous material (CA-1 and CA-2), respectively. The prepared carbons were characterized by thermal analysis with heating rate of 10 °C min−1 in N2 atmosphere, showing two main stages: the first one close to 150 °C for biochar samples and the second one in the range of 150–600 °C for the same biochar samples, X-ray diffraction (XRD, peaks at 2θ = 28.41°; 40.50°; 44.52°; 50.29° and 58.61° for CA-1 and 2θ = 28.27°; 40.45°; 44.60°; 50.15° and 58.63° for CA-2), scanning electron microscopy (SEM) with energy-dispersive X-ray (EDX) and Fourier transform infrared spectroscopy (FTIR, showing peaks at 3675, 2926, 1559, 1364, 1026 and 916 cm−1 for the biochar samples). CO2 capture was performed by thermogravimetric running test. The samples showed similar physico-chemical characteristics, but the CA-2 sample showed better adsorption performance (1.22 mmol CO2 g−1). Heat treatment in an inert atmosphere and chemical activation of biomass are recommended for obtaining carbonaceous material for use in carbon dioxide adsorption processes.










Similar content being viewed by others
References
Fiuza RA, Medeiros de Jesus Neto R, Correia LB, Carvalho Andrade HM. Preparation of granular activated carbons from yellow mombin fruit stones for CO2 adsorption. J Environ Manag. 2015;161:198–205.
Petrescu L, Bonalumi D, Valenti G, Cormos AM, Cormos CC. Life cycle assessment for supercritical pulverized coal power plants with post-combustion carbon capture and storage. J Clean Prod. 2017;157:10–21.
Intergovernmental Panel on Climate Change. Climate change 2007: synthesis report. In: Core Writing Team, Pachauri RK, Reisinger A, editors. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: Intergovernmental Panel on Climate Change; 2007.
Peng Y, Zhao B, Li L. Advance in post-combustion CO2 capture with alkaline solution: a brief review. Energy Proced. 2012;14:1515–22.
Cuéllar-Franca RM, Azapagic A. Carbon capture, storage and utilisation technologies: a critical analysis and comparison of their life cycle environmental impacts. J CO2 Util. 2015;9:82–102.
Heidari A, Younesi H, Rashidi A, Ghoreyshi AA. Adsorptive removal of CO2 on highly microporous activated carbons prepared from Eucalyptus camaldulensis wood: effect of chemical activation. J Taiwan Inst Chem Eng. 2014;45:579–88.
He X, Liu J, Jiang Y, Yaseen M, Guan H, Sun J, et al. ‘Useful’ template synthesis of N-doped acicular hollow porous carbon/carbon-nanotubes for enhanced capture and selectivity of CO2. Chem Eng J. 2019;361:278–85.
Zhou F, Tien HN, Dong Q, Xu WL, Li H, Li S, et al. Ultrathin, ethylenediamine-functionalized graphene oxide membranes on hollow fibers for CO2 capture. J Memb Sci. 2019;573:184–91.
Sawant SY, Munusamy K, Somani RS, John M, Newalkar BL, Bajaj HC. Precursor suitability and pilot scale production of super activated carbon for greenhouse gas adsorption and fuel gas storage. Chem Eng J. 2017;315:415–25.
Furtado AMB, Wang Y, Levan MD. Carbon silica composites for sulfur dioxide and ammonia adsorption. Microporous Mesoporous Mater. 2013;165:48–54.
Niazi L, Lashanizadegan A, Sharififard H. Chestnut oak shells activated carbon: preparation, characterization and application for Cr (VI) removal from dilute aqueous solutions. J Clean Prod. 2018;185:554–61.
Song Y, Liu T, Qian F, Zhu C, Yao B, Duoss E, et al. Three-dimensional carbon architectures for electrochemical capacitors. J Colloid Interface Sci. 2018;509:529–45.
Kwiatkowski M, Broniek E. An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation. Colloids Surf A Physicochem Eng Asp. 2017;529:443–53.
Zahedifar M, Moosavi AA. Assessing cadmium availability of contaminated saline-sodic soils as influenced by biochar using the adsorption isotherm models. Arch Agron Soil Sci. 2020;66:1735–52.
Zahedifar M. Effect of biochar on cadmium fractions in some polluted saline and sodic soils. Environ Manag. 2020;66:1133–41.
Zahedifar M, Moosavi AA. Modeling desorption kinetics of the native and applied zinc in biochar-amended calcareous soils of different land uses. Environ Earth Sci. 2017;76:567.
Moradi-Choghamarani F, Moosavi AA, Baghernejad M. Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J Therm Anal Calorim. 2019;138:331–42.
Moradi-Choghamarani F, Moosavi AA, Sepaskhah AR, Baghernejad M. Physico-hydraulic properties of sugarcane bagasse-derived biochar: the role of pyrolysis temperature. Cellulose. 2019;26:7125–43.
Bodunrin MO, Burman NW, Croft J, Engelbrecht S, Goga T, Ladenika AO, et al. The availability of life-cycle assessment, water footprinting, and carbon footprinting studies in Brazil. Int J Life Cycle Assess. 2018;23:1701–7.
Caldeira-Pires A, Benoist A, da Luz SM, Silverio VC, Silveira CM, Machado FS. Implications of removing straw from soil for bioenergy: an LCA of ethanol production using total sugarcane biomass. J Clean Prod. 2018;181:249–59.
Du C, Ugaya C, Freire F, Dias LC, Clift R. Enriching the results of screening social life cycle assessment using content analysis: a case study of sugarcane in Brazil. Int J Life Cycle Assess. 2019;24:781–93.
Paulo J, Morais S, De Freitas M, José R, Marconcini M. Procedimentos para Análise Lignocelulósica, 1st ed. Campina Grande; 2010 [cited 2021 Apr 22]. http://www.cnpa.embrapa.br
Morais LC, Maia AAD, Guandique MEG, Rosa AH. Pyrolysis and combustion of sugarcane bagasse. J Therm Anal Calorim. 2017;129:1813–22.
Correia LB, Fiuza RA, de Andrade RC, Andrade HMC. CO2 capture on activated carbons derived from mango fruit (Mangifera indica L.) seed shells: A TG study. J Therm Anal Calorim. 2018;131:579–86.
Labus K, Gryglewicz S, Machnikowski J. Granular KOH-activated carbons from coal-based cokes and their CO2 adsorption capacity. Fuel. 2014;118:9–15.
Król M, Gryglewicz G, MacHnikowski J. KOH activation of pitch-derived carbonaceous materials: effect of carbonization degree. Fuel Process Technol. 2011;92:158–65.
Singh G, Ismail IS, Bilen C, Shanbhag D, Sathish CI, Ramadass K, et al. A facile synthesis of activated porous carbon spheres from D-glucose using a non-corrosive activating agent for efficient carbon dioxide capture. Appl Energy. 2019;255: 113831.
Elmouwahidi A, Bailón-García E, Pérez-Cadenas AF, Maldonado-Hódar FJ, Carrasco-Marín F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim Acta. 2017;229:219–28.
Hadoun H, Sadaoui Z, Souami N, Sahel D, Toumert I. Characterization of mesoporous carbon prepared from date stems by H3PO4 chemical activation. Appl Surf Sci. 2013;280:1–7.
Vargas AMM, Cazetta AL, Garcia CA, Moraes JCG, Nogami EM, Lenzi E, et al. Preparation and characterization of activated carbon from a new raw lignocellulosic material: Flamboyant (Delonix regia) pods. J Environ Manag. 2011;92:178–84.
Huang G, Liu Y, Wu X, Cai J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. Carbon N Y. 2019;153:804–5.
Zhang Q, Han K, Li S, Li M, Li J, Ren K. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors. Nanoscale. 2018;10:2427–37.
Kim YK, Kim GM, Lee JW. Highly porous N-doped carbons impregnated with sodium for efficient CO2 capture. J Mater Chem A. 2015;3:10919–27.
Liu SH, Huang YY. Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J Clean Prod. 2018;175:354–60.
Liu B, Li H, Ma X, Chen R, Wang S, Li L. The synergistic effect of oxygen-containing functional groups on CO2 adsorption by the glucose-potassium citrate-derived activated carbon. RSC Adv. 2018;8:38965–73.
Masoomi MY, Stylianou KC, Morsali A, Retailleau P, Maspoch D. Selective CO2 capture in metal-organic frameworks with azine-functionalized pores generated by mechanosynthesis. Cryst Growth Des. 2014;14:2092–6.
Xu Z, Guo Z, **ao X, Zeng P, Xue Q. Effect of inorganic potassium compounds on the hydrothermal carbonization of Cd-contaminated rice straw for experimental-scale hydrochar. Biomass Bioenergy. 2019;130:105357.
Torrellas SÁ, García Lovera R, Escalona N, Sepúlveda C, Sotelo JL, García J. Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem Eng J. 2015;279:788–98.
Guo Y, Tan C, Sun J, Li W, Zhang J, Zhao C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem Eng J. 2020;381:122736.
Islam MA, Tan IAW, Benhouria A, Asif M, Hameed BH. Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem Eng J. 2015;270:187–95.
Hsu SH, Huang CS, Chung TW, Gao S. Adsorption of chlorinated volatile organic compounds using activated carbon made from Jatropha curcas seeds. J Taiwan Inst Chem Eng. 2014;45:2526–30.
Ma C, Mao Y, Xu P, Liu J, Song X. Preparation and characterization of high specific area activated carbon by KOH/NaOH activation from abandoned capsicum straw. J Funct Biomater. 2013;44:704–8.
Zhang B, Xu P, Qiu Y, Yu Q, Ma J, Wu H, et al. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem Eng J. 2015;263:1–8.
Dobele G, Dizhbite T, Gil MV, Volperts A, Centeno TA. Production of nanoporous carbons from wood processing wastes and their use in supercapacitors and CO2 capture. Biomass Bioenergy. 2012;46:145–54.
Qajar A, Daigle H, Prodanović M. The effects of pore geometry on adsorption equilibrium in shale formations and coal-beds: Lattice density functional theory study. Fuel. 2016;163:205–13.
Yang M, Guo L, Hu G, Hu X, Xu L, Chen J, et al. Highly cost-effective nitrogen-doped porous coconut shell-based CO2 sorbent synthesized by combining ammoxidation with KOH activation. Environ Sci Technol. 2015;49:7063–70.
Xu L, Guo L, Hu G, Chen J, Hu X, Wang S, et al. Nitrogen-doped porous carbon spheres derived from d-glucose as highly-efficient CO2 sorbents. RSC Adv. 2015;5:37964–9.
Jiménez V, Ramírez-Lucas A, Díaz JA, Sánchez P, Romero A. CO2 capture in different carbon materials. Environ Sci Technol. 2012;46:7407–14.
Creamer AE, Gao B, Zhang M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J. 2014;249:174–9.
Zhou L, Feng QY, Qin Y. Thermodynamic analysis of competitive adsorption of CO2 and CH4 on coal matrix. J China Coal Soc. 2011;36:1307–11.
Younas M, Sohail M, Kong LL, Bashir MJK, Sethupathi S. Feasibility of CO2 adsorption by solid adsorbents: a review on low-temperature systems. Int J Environ Sci Technol. 2016;13:1839–60.
Yu L, Kanezashi M, Nagasawa H, Tsuru T. Role of amine type in CO2 separation performance within amine functionalized silica/organosilica membranes: a review. Appl Sci. 2018;8.
Su F, Lu C, Cnen W, Bai H, Hwang JF. Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci Total Environ. 2009;407:3017–23.
Borchardt L, Zhu QL, Casco ME, Berger R, Zhuang X, Kaskel S, et al. Toward a molecular design of porous carbon materials. Mater Today. 2017;20:592–610.
Deng S, Wei H, Chen T, Wang B, Huang J, Yu G. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem Eng J. 2014;253:46–54.
Hong SM, Jang E, Dysart AD, Pol VG, Lee KB. CO2 capture in the sustainable wheat-derived activated microporous carbon compartments. Sci Rep. 2016;6:1–10.
Plaza MG, Pevida C, Arias B, Fermoso J, Casal MD, Martín CF, et al. Development of low-cost biomass-based adsorbents for postcombustion CO2 capture. Fuel. 2009;88:2442–7.
Shafeeyan MS, Daud WMAW, Houshmand A, Arami-Niya A. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: effect of pre-oxidation. Appl Surf Sci. 2011;257:3936–42.
Alhassan M, Andrew I, Auta M, Umaru M, Garba MU, Isah AG, et al. Comparative studies of CO2 capture using acid and base modified activated carbon from sugarcane bagasse. Biofuels. 2018;9:719–28.
Acknowledgements
The authors would like to gratefully acknowledge the São Paulo Research Foundation (FAPESP) (process no: 2018/03138-7) and Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support, São Paulo State University (UNESP Sorocaba-ICTS) for the opportunity to develop the project, the Laboratory of Activated Carbon (LCA) of Federal University of Paraiba (UFPB) for performing CO2 capture analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peres, C.B., Rosa, A.H. & de Morais, L.C. CO2 adsorption of bagasse waste feedstock using thermogravimetric analyses. J Therm Anal Calorim 147, 5973–5984 (2022). https://doi.org/10.1007/s10973-021-10949-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10949-2