Abstract
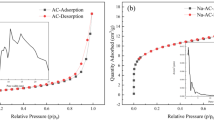
With the wide application of beryllium globally, industrial wastewater has rapidly increased. Previously, adsorption was effective in treating this issue. However, most adsorbents have a poor removal rate, primarily in the low adsorption capacity. To remove Be from industrial wastewater and overcome the disadvantages of low adsorption capacity and poor removal rate of existing adsorbents, typical agricultural waste lotus leaf was used to prepare Al-activated carbon (Al-AC) by the impregnation-calcination modification of Al(NO3)3. The theoretical maximum adsorption capacity of Al-AC was 32.86 mg/g. Langmuir, Freundlich, and Temkin models were used to thermodynamically analyze Al-AC, and adsorption thermodynamics demonstrated that the adsorption reaction of Al-AC was endothermic. Through characterization analysis, the specific surface area of the modified AC increased from 4.3573 to 155.87 m2/g. This study provides a new approach to preparing and modifying AC and a new method for removing Be from industrial wastewater.
Similar content being viewed by others
References
T. **angyuan, X. Lei, W. Rucheng, Z. Rongqing, H. Huan and L. Chen, J. Nan**g University (Natural Science), 56, 815 (2020).
L. Chengxing, Engineering study on biological treatment of beryllium-containing wastewater, Master dissertation, Central South University (2010).
M. Tanveer and L. Wang, Plant Physiol. Bioch., 139, 691 (2019).
M. L. Chiarappa-Zucca, R. C. Finkel, R. E. Martinelli, J. E. McAninch, D._O. Nelson and K. W. Turteltaub, Chem. Res. Toxicol., 17, 1614 (2004).
B. Vladimír, V. Elena and V. Symon Karel, Environ. Res., 22, 439 (1980).
Z. Minglong, C. Liyuan, M. **aobo, L. Qingzhu and W. Shunwen, Technol. Water Treat., 32, 45 (2006).
L. Phillip, J. Environ, Sci. Heal A., 25, 21 (2008).
L. C. Robles and A. J. Aller, Quim. Anal., 15, 21 (1995).
J. Ke, Z. Kanggen and Y. Youcai, Nonferrous Metal (Smelting Section), 06, 83 (2018).
X. Xuejun and L. Yanhui, Chinese J. Rare Metals., S1, 59 (2007).
C. Hewen, Z. **aojia, X. Haiying and Y. **aorong, Water Technol., 06, 16 (2011).
W. Minzhan, Z. Zhengke, C. Sili, C. Sha, G. Qingwei and W. **song, Technol. Water Treat., 47, 78 (2021).
K. Ersin, B. Sezgin and Y. Mehmet, Food Addit Contam Part A Chem. Anal. Control Expo. Risk Assess., 28, 455 (2011).
D. Yongbo, X. Lei, Q. Zhimin and S. Honglan, Chemosphere, 262, 127940 (2021).
L. **anchun, W. Huanran and S. Gege, Int. J. Hydrogen Energ., 144, 25265 (2019).
S. Norouzi, M. Heidari, V. Alipour and K. Dindarloo, Bioresour. Technol., 258, 48 (2018).
F. Sabrina. Lütke, V. Andrei. Igansi, L. Pegoraro and R. s. Tito, J. Environ. Chem. Eng., 17, 103396 (2019).
L. Lin, L. Suqin and L. Junxin, J. Hazard. Mater., 192, 683 (2011).
Y. Kuang, Z. ** and Z. Shaoqi, Water, 12, 587 (2020).
A. S. Zulaicha, J. Phys. Conf. Ser., 1, 1751 (2021).
L. Youji, L. **g, M. Mingyuan and Y. Wenbin, Sci. China Ser. B., 52, 1113 (2009).
P. Karthikeyan and S. Meenakshi, J. Mol. Liq., C, 296, 111766 (2019).
R. Zhijun, J. Biao, Z. Guangming and L. Longyi, Chemosphere, 262, 127895 (2021).
K. A. Al-Saad, M. A. Amr, D. T. Hadi, R. S. Arar, M. M. AL-Sulaiti, T. A. Abdulmalik, N. M. Alsahamary and J. C. Kwak, Arab. J. Nucl. Sci. Appl., 45, 335 (2012).
B. Kotaro, K. Naoki, S. Saki and M. Hideaki, Anal. Sci., 11, 1067 (2014).
Y. Yücel and S. Göycincik, Waste Biomass Valori, 6, 1077 (2015).
E. Maria Iannicelli-zubiani, P. Gallo Stampino, C. Cristiani and G. Dotelli, Chem. Eng. J., 341, 75 (2018).
O. Pezoti, A. L. Cazetta, K. C. Bedin, L. S. Souza, A. C. Martins, T. L. Silva and V. C. Almeida, Chem. Eng. J., 288, 778 (2016).
A. C. Martins, O. Pezoti, A. L. Cazetta and V. C. Almeida, Chem. Eng. J., 288, 291 (2015).
P. Senthil Kumar, S. Ramalingam, S. D. Kirupha and S. Sivanesan, Chem. Eng. J., 167, 122 (2010).
A. Oberlintner, V. Shvalya, A. Vasudevan, D. Vengust, B. Likozar, U. Cvelbar and U. Novak, Appl. Surf. Sci., 581, 152276 (2022).
M. Thommes, Pure Appl. Chem., 87, 1051 (2016).
L. Weifeng, Z. Jian, Z. Chenglu and L. Ye, Chem. Eng. J., 162, 677 (2010).
Z. Heshan, G. Wanqian, L. Shuo and C. Jo-shu, Bioresour. Technol., 244, 1456 (2017).
T. Watanabe, Y. Miki, T. Masuda, H. Kanai, S. Hosokawa, K. Wada and M. Inoue, Appl. Catal. A-gen., 396, 140 (2011).
T. Depci, Chem. Eng. J., 181, 467 (2012).
I. Abdulkadir, B. S. Mohammed, M. S. Liew and M. M. A. Wahab, Case Stud. Constr. Mat., 14, E00525 (2021).
S. Çam Kaynar and Ü. H. Kaynar, Nucl. Sci. Tech., 30, 75 (2019).
S. Fang, S. Wei-ling, S. Hai-mei and N. **-ren, Chem. Eng. J., 172, 783 (2011).
V. S. Savenko, Russ. J. Inorg. Chem., 52, 465 (1965).
Y. Chenhui, Z. Yongqing, D. Meimei, D. **aodong and H. Shaobin, Chem. Eng. J., 362, 262 (2019).
Z. Peizhen, Z. **aoxiao, Y. **angru, X. Ruyue and H. Lujia, Bioresour. Technol., 331, 125013 (2021).
C. Yaoning, L. Meiling, L. Yuan**, L. Yihuan, C. Yanrong, L. Hui and L. Chen, Bioresour. Technol., 321, 12443 (2021).
H. Karaca, E. Atıntığ, D. Türker and M. Teker, J. Disper. Sci. Technol., 39, 1800 (2018).
F. Mashkoor, A. Nasar, I. Abdullah and M. Asiri, Sci. Rep-uk., 8, 8314 (2018).
M. Shaban, M. R. Abukhadra, M. G. Shahien and A. A. P. Khan, Environ. Sci. Pollut. R., 24, 18135 (2017).
C. Huayi, L. Wenyan, W. **** and Z. Yulong, Bioresour. Technol., 292, 12198 (2019).
Z. **n, L. Yaru, W. Mengru, P. Yao, H. Zhenbing, H. Mian and C. Zhihua, Bioresour. Technol., 320, 124264 (2021).
Z. **aogang, H. **nyue, P. Hao, W. **g and L. Sihao, Bioresour. Technol., 334, 125238 (2021).
R. Gang, Y. Yan, Y. Zhixin, D. Yaomin and L. **aoyi, J. Saf. Environ., 16, 208 (2016).
M. O. Abd El-magied, A. Mansour, F. A. Al Ghani Alsayed and S. Abd Eldayem, J. Disper. Sci. Technol., 39, 1597 (2018).
N. N. Basargin and O. V. Miroshnichenko, Russ. J. Inorg. Chem., 57, 758 (2012).
A. B. Petriciolet, D. I. M. Castillo and H. E. R. Ávila, Adsorption processes for water treatment and purification, Springer, Publications, Berlin (2017).
M. Hadi, M. R. Samarghandi and G. Mckay, Chem. Eng. J., 160, 408 (2010).
A. S. Singha and A. Guleria, J. Environ. Chem. Eng., 2, 1456 (2014).
S. Srivastava, S. B. Agrawal and M. K. Mondal, Korean. J. Chem. Eng., 33, 567 (2016).
S. Mirzaeei, F. H. Pirhayati, G. Mohammadi, E. Rahimpour, F. Martinez and A. Jouyban, Phys. Chem. Liq., 57, 788 (2019).
L. Jiayang, H. Changwei and H. Qingguo, Bioresour. Technol., 271, 487 (2018).
L. Yu and L. Ya-juan, Sep. Purif. Technol., 61, 229 (2007).
M. Barkat, D. Nibou, S. Chegrouche and A. Mellah, Chem. Eng. Process., 48, 38 (2007).
Acknowledgements
We thank the following funding agencies for supporting this work: Foundation of State Key Laboratory of Nuclear Resources and Environment (2020NRE02). Research on characteristic properties of typical radioactive solid waste and radiation protection regulation technology and operation management mechanism (2019YFC1907701).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, X., Su, Y., Wang, H. et al. Modification of activated carbon from agricultural waste lotus leaf and its adsorption mechanism of beryllium. Korean J. Chem. Eng. 40, 255–266 (2023). https://doi.org/10.1007/s11814-022-1251-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-022-1251-8