Abstract
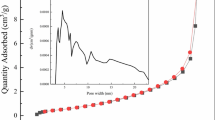
A modified material of activated carbon by alkali leaching pretreatment (NaOH-AC) for adsorbing beryllium was reported. The batch test results showed that the maximum adsorption capacity of beryllium by NaOH-AC was 40.59 mg/g. The pH value of the treated solution was 6, which met the discharge standards of industrial wastewater. The results of the BET analysis showed that NaOH-AC had a high specific surface area and a small pore size. The kinetic results showed that the process of NaOH-AC adsorption of beryllium was mainly controlled by chemical adsorption but also by physical adsorption and intraparticle diffusion. The thermodynamic results showed that the adsorption process was a spontaneous endothermic reaction. The comparative characterization of beryllium adsorption by NaOH-AC showed that beryllium existed on the surface of activated carbon in the form of Be(OH)2.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Abd El-Magied, M. O., Mansour, A., Al Ghani Alsayed, F. A., Abd Eldayem, S. (2018). Biosorption of beryllium from aqueous solutions onto modified chitosan resin: Equilibrium, kinetic and thermodynamic study. Journal of Dispersion Science and Technology, (11). https://doi.org/10.1080/01932691.2018.1452757
Al Isawi, W. A., Zeller, M., & Mezei, G. (2022). Supramolecular incarceration and extraction of tetrafluoroberyllate from water by nanojars. Inorganic Chemistry. https://doi.org/10.1021/acs.inorgchem.2c01198
Aller, A. J., & Robles, L. C. (1995). Immobilized bacterial cells as bio-sorbent for toxic elements (1995). Quimica Analitica, 15, 21–31.
Arampatzidou, A., Voutsa, D., & Deliyanni, E. (2018). Removal of bisphenol A by Fe-impregnated activated carbons. Environmental Science and Pollution Research, 25(26), 25869–25879. https://doi.org/10.1007/s11356-018-2652-4
Barkat, M., Nibou, D., Chegrouche, S., & Mellah, A. (2009). Kinetics and thermodynamics studies of chromium(VI) ions adsorption onto activated carbon from aqueous solutions. Chemical Engineering & Processing: Process Intensification., 48, 38–47. https://doi.org/10.1016/j.cep.2007.10.004
Chen H, Zhang X, **e H, et al. (2011). Removal of Beryllium from water by chemical precipitation[J]. Water Technology, 5(06), 16–18.
Chen, Y., Li, M., Li, Y., Liu, Y., Chen, Y., Li, H., Li, L., Xu, F., Jiang, H., & Chen, L. (2021). Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms[J]. Bioresource Technology, 321, 124413. https://doi.org/10.1016/j.biortech.2020.124413
Chiarappa-zucaa, M. L., Finkel, R. C., Martinelli R. E., et al. (2004). Measurement of beryllium in biological samples by accelerator mass spectrometry: Applications for studying chronic beryllium disease.[J]. Chemical Research in Toxicology, 17, (12), 1614–20. https://doi.org/10.1021/tx049883o
Cloeren, M., Dement, J., Gaitens, J., Hines, S., Diaz, L., Tembunde, Y., ... & Beryllium in Construction. (2022). Beryllium disease among construction trade workers at Department of Energy nuclear sites: A follow‐up. American Journal of Industrial Medicine, 65(9), 708–720. https://doi.org/10.1002/ajim.23411
Gan, J., Zhang, L., Wang, Q., **n, Q., Hu, E., Lei, Z., ... & Wang, H. (2023). Synergistic action of multiple functional groups enhanced uranium extraction from seawater of porous phosphorylated chitosan/coal-based activated carbon composite sponge. Desalination, 545, 116154. https://doi.org/10.1016/j.desal.2022.116154
Gang, R., Yan, Y., et al. (2016). Adsorption of beryllium (Be) from wastewater by chitosan modified zeolite [J]. Journal of Safety and Environment, 16(06), 208–213. https://doi.org/10.1016/J.BIORTECH.2021.125238
Gatewood, P. L. & Sneddon, J. (2008). Removal and recovery of beryllium in waters by chlorella vulgaris. Journal of Environmental Science & Health Part A, (1). https://doi.org/10.1080/10934529009375537
Ho, H. P., Kasinathan, P., Kim, J., Lee, D., & Woo, H. C. (2016). Deep desulfurization of fuel gas by adsorption on Cu-impregnated activated carbons in practical conditions. Korean Journal of Chemical Engineering, 33(6), 1908–1916. https://doi.org/10.1007/s11814-016-0018-5
Iannicelli-Zubiani, E. M., Stampino, P. G., Cristiani, C., Dotelli, G. (2018). Enhanced lanthanum adsorption by amine modified activated carbon[J]. Chemical Engineering Journal, 341. https://doi.org/10.1016/j.cej.2018.01.154
Isaacs, M. A., Davies-Jones, J., Davies, P. R., Guan, S., Lee, R., Morgan, D. J., & Palgrave, R. (2021). Advanced XPS characterization: XPS-based multi-technique analyses for comprehensive understanding of functional materials. Materials Chemistry Frontiers, 5(22), 7931–7963. https://doi.org/10.1039/d1qm00969a
Kilinc, E., Bakipdere, S., Yaman, M (2011) Trace level determination of beryllium in natural and flavored mineral waters after pre-concentration using activated carbon.[J]. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment, 4(28):455–460. https://doi.org/10.1080/19440049.2011.551946
Li, X., Wang, H., et al. (2019). Low temperature reduction of NO by activated carbons impregnated with Fe based catalysts(Article)[J]. International Journal of Hydrogen Energy, 44(46), 25265–25275. https://doi.org/10.1016/j.ijhydene.2019.04.008
Liu, Y., & Liu, Y. (2008). Biosorption isotherms, kinetics and thermodynamics. Separation and Purification Technology, 61, 229–242. https://doi.org/10.1016/j.seppur.2007.10.002
Liu, W., Zhang, J., Zhang, C., Wang, Y., & Li, Y. (2010). Adsorptive removal of Cr (VI) by Fe-modified activated carbon prepared from Trapa natans husk. Chemical Engineering Journal, 162, 677–684. https://doi.org/10.1016/j.cej.2010.06.020
Liu, P., Wu, Z., Sun, Z., & Ye, J. (2018). Comparison study of naphthalene adsorption on activated carbons prepared from different raws. Korean Journal of Chemical Engineering, 35(10), 2086–2096. https://doi.org/10.1007/s11814-018-0124-7
Liu, J., Hu, C., & Huang, Q. (2018). Adsorption of Cu 2+, Pb 2+, and Cd 2+ onto oiltea shell from water[J]. Bioresource Technology, 271, 487–491. https://doi.org/10.1016/j.biortech.2018.09.040
Martins, A. C., Pezoti, O., et al. (2015). Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies[J]. Chemical Engineering Journal, 260, 291–299. https://doi.org/10.1016/j.cej.2014.09.017
Minglong, Z., Liyuan, C., et al. (2006). Adsorption and desorption of beryllium in wastewater by activated sludge[J]. Technology of Water Treatment, (02), 45–48. https://doi.org/10.16796/j.cnki.1000-3770.2006.02.014
Minzhan, W., Zhengke, Z., Sili, C., et al. (2021). Experimental study on screening of emergency adsorption materials for sudden beryllium pollution in water body. Technology of Water Treatment, 47(05), 78–82. https://doi.org/10.16796/j.cnki.1000-3770.2021.05.016
Mirzaeei, S., Pirhayati, F. H., Mohammadi, G., Rahimpour, E., Martinez, F., & Jouyban, A. (2019). Solubility of minoxidil in binary mixture of ethanol+ water at various temperatures. Physics and Chemistry of Liquids, 57(6), 788–799. https://doi.org/10.1080/00319104.2018.1528596
Mondal, S., & Majumder, S. K. (2019). Synthesis of phosphate functionalized highly porous activated carbon and its utilization as an efficient copper (II) adsorbent. Korean Journal of Chemical Engineering, 36(5), 701–712. https://doi.org/10.1007/s11814-019-0260-8
Petanová, J., & Bencko, V. (2020). Health aspects of exposure to emissions from burning coal of high beryllium content: Interactions with the immune system[J]. Central European Journal of Public Health, 128(3), 198–201. https://doi.org/10.21101/cejph.a5851
Pezoti, O.,Cazetta, A..L. et al. (2016). NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies[J]. Chemical Engineering Journal, 288, 778–788. https://doi.org/10.1016/j.cej.2015.12.042
Polowczyk, I., Bastrzyk, A., & Fiedot, M. (2016). Protein-mediated precipitation of calcium carbonate. Materials, 9(11), 944. https://doi.org/10.3390/ma9110944
Senthil Kumar, P., Dinesh Kirupha, S., et al. (2011). Adsorption behavior of nickel(II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design[J]. Chemical Engineering Journal, 167, 122–131. https://doi.org/10.1016/j.cej.2010.12.010
Singha, A. S., & Guleria, A. (2014). Application of vinyl monomers functionalized cellulosic biopolymer for removal of dissolved toxic metal ions from polluted water samples[J]. Journal of Environmental Chemical Engineering, 2(3), 1456–1466. https://doi.org/10.1016/j.jece.2014.07.014
Sun, F., Sun, W-L., Sun, H-M., **-Ren, N. (2011). Biosorption behavior and mechanism of beryllium from aqueous solution by aerobic granule. Chemical Engineering Journal, (2). https://doi.org/10.1016/j.cej.2011.06.062
Thommes, M. (2016). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Chemistry International the News Magazine of IUPAC, 38(1), 1051–1069. https://doi.org/10.1515/pac-2014-1117
Zhang, P., Zhang, X., Yuan, X., **e, R., & Han, L. (2021). Characteristics, adsorption behaviors, Cu(II) adsorption mechanisms by cow manure biochar derived at various pyrolysis temperatures[J]. Bioresource Technology, 331, 125013.
Zhang, X., Lv, L., Qin, Y., & Chen, Z. (2018). Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresource Technology. https://doi.org/10.1016/j.biortech.2018.01.145
Zhao, X., Su, Y., Hao, X., et al. (2023c). Effect of mechanical−chemical modification on adsorption of beryllium by calcite. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-023-27275-9
Zhao, X., Su, Y., Lei, Z., Wang, H., Hu, E., Hu, F., ... & Hao, X. (2022). Adsorptive removal of beryllium by Fe-modified activated carbon prepared from lotus leaf. Environmental Science and Pollution Research, 1–14. https://doi.org/10.1007/s11356-022-23415-9
Zhao, X., Su, Y., Wang, H., Lei, Z., Hu, E., Hu, F., ... & Hao, X. (2023a). Modification of activated carbon from agricultural waste lotus leaf and its adsorption mechanism of beryllium. Korean Journal of Chemical Engineering, 40(1), 255–266. https://doi.org/10.1007/s11814-022-1251-8
Zhao, X., Dong, S., Wang, H., Hu, E., Hu, F., Lei, Z., Wang, Q., Zhou, C., Fan, S., Liu, X., Hao, X., Su, Y. (2023b). Preparation of porous calcium carbonate biochar and its beryllium adsorption performance. Journal of Environmental Chemical Engineering, 110102. https://doi.org/10.1016/j.jece.2023.110102
Zheng, X., He, X., Peng, H., Wen, J., & Lv, S. (2021). Efficient adsorption of ciprofloxacin using Ga2S3/S-modified biochar via the high-temperature sulfurization[J]. Bioresource Technology, 334, 125238.
Zheng, H., Wanqian, G., Li, S., Chang, J-S. (2017). Adsorption of p-nitrophenols (PNP) on microalgal biochar: Analysis of high adsorption capacity and mechanism. Bioresource Technology, (Part 2). https://doi.org/10.1016/j.biortech.2017.05.025
Funding
This study was supported by the Foundation of State Key Laboratory of Nuclear Resources and Environment (2020NRE02), research on characteristic properties of typical radioactive solid waste and radiation protection regulation technology and operation management mechanism (Ministry of Science and Technology of China, 2019YFC1907701), and Scientific Research Project of Education Department of Hunan (Project No. 21C0297),
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Xu Zhao, Boyuan Zheng, Zhiwu Lei, Yucheng Su, Hongqiang Wang, Eming Hu, Pengfei Hu, Fang Hu, Qingliang Wang, Chunze Zhou, and Hongyang **a. The first draft of the manuscript was written by Xu Zhao, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. NaOH-AC was used for the first time to treat beryllium-containing wastewater.
2. The adsorption capacity of NaOH-AC was 40.63 mg/g.
3. Alkaline leaching can improve the treatment capacity of high concentration wastewater.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Zheng, B., **a, H. et al. Effective Removal of Beryllium from Industrial Wastewater by Alkali-Leaching Activated Carbon. Water Air Soil Pollut 234, 568 (2023). https://doi.org/10.1007/s11270-023-06577-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06577-1