Abstract
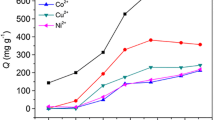
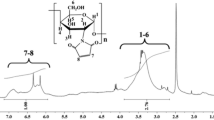
Using Pearson’s hard and soft acids and bases (HSAB) principle as the design guideline, a novel chitosan/poly(ethylene glycol)/poly(acrylic acid) (CS/PEG/PAA) hydrogel adsorbent containing hard Lewis base adsorption site (i.e., amino group, hydroxyl group, ether linkage, carboxylate ions) was prepared by glow-discharge electrolysis plasma (GDEP) technique and then applied for the selective adsorption of Pb2+. A copolymerization mechanism initiated by GDEP was proposed. The structure, thermal stability, and morphology of CS/PEG/PAA adsorbent were characterized by FT-IR, thermogravimetry/differential thermogravimetry (TG/DTG), and SEM. The effects of the solution pH and contact time on the single-component adsorption were examined by batch experiments. Competitive adsorptions of Pb2+ and Cd2+ were carried out under equal mass concentration and equal molar concentration. In addition, regeneration and reusability of adsorbent were also investigated in detail. The results showed that the maximum adsorption capacities for Pb2+ and Cd2+ are 431.7 and 265.0 mg g−1, respectively. The kinetic behaviors of the adsorption and the fourth desorption for single component and the competitive adsorption for Pb2+ follow the pseudo-second-order model with pH = 4.8. The CS/PEG/PAA adsorbent has good adsorption selectivity for Pb2+ with the coexistence of Cd2+. The adsorbent displays excellent regeneration and reusability in the EDTA-4Na solution.












Similar content being viewed by others
References
Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater B 122:61–170. doi:10.1016/j.jhazmat.2005.03.024
Liu YM, Ju XJ, **n Y, Zheng WC, Wang W, Wei J, **e R, Liu Z, Chu LY (2014) A novel smart microsphere with magnetic core and ion-recognizable shell for Pb2+ adsorption and separation. ACS Appl Mater Interfaces 6:9530–9542. doi:10.1021/am501919j
Li X, Li Y, Ye Z (2011) Preparation of macroporous bead adsorbents based on poly(vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chem Eng J 178:60–68. doi:10.1016/j.cej.2011.10.012
Gandhi MR, Meenakshi S (2012) Preparation and characterization of silica gel/chitosan composite for the removal of Cu(II) and Pb(II). Int J Biol Macromol 50:650–657. doi:10.1016/j.ijbiomac.2012.01.012
Laus R, Costa TG, Szpoganicz B, Fávere VT (2010) Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J Hazard Mater 183:233–241. doi:10.1016/j.jhazmat.2010.07.016
Zhou G, Luo J, Liu C, Chu L, Ma J, Tang Y, Zeng Z, Luo S (2016) A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res 89:151–160. doi:10.1016/j.watres.2015.11.053
Yu J, Li Y, Lu QF, Zheng JD, Yang SX, ** F, Wang QZ, Yang W (2016) Synthesis, characterization and adsorption of cationic dyes by CS/P(AMPS-co-AM) hydrogel initiated by glow-discharge-electrolysis plasma. Iran Polym J 25:423–435. doi:10.1007/s13726-016-0434-8
Yu J, Zhang HT, Li Y, Lu QF, Wang QZ, Yang W (2016) Synthesis, characterization, and property testing of PGS/P(AMPS-co-AM) superabsorbent hydrogel initiated by glow-discharge electrolysis plasma. Colloid Polym Sci 294:257–270. doi:10.1007/s00396-015-3751-0
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74. doi:10.1016/j.seppur.2003.10.004
Wu FC, Tseng RL, Juang RS (2010) A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J Environ Manag 91:798–806. doi:10.1016/j.jenvman.2009.10.018
Zhao F, Repo E, Sillanpää M, Meng Y, Yin D, Tang WZ (2015) Green synthesis of magnetic EDTA- and/or DTPA-cross-linked chitosan adsorbents for highly efficient removal of metals. Ind Eng Chem Res 54:1271–1281. doi:10.1021/ie503874x
Paulino AT, Belfiore LA, Kubota LT, Munizd EC, Almeida VC, Tambourgi EB (2011) Effect of magnetite on the adsorption behavior of Pb(II), Cd(II), and Cu(II) in chitosan-based hydrogels. Desalination 275:187–196. doi:10.1016/j.desal.2011.02.056
Kyzas GZ, Siafaka PI, Lambropoulou DA, Lazaridis NK, Bikiaris DN (2014) Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for Pb(II) and Cd(II) uptake. Langmuir 30:120–131. doi:10.1021/la402778x
Zhao F, Repo E, Yin D, Sillanpää M (2013) Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: kinetics and isotherms. J Colloid Interface Sci 409:174–182. doi:10.1016/j.jcis.2013.07.062
Khan A, Badshah S, Airoldi C (2011) Biosorption of some toxic metal ions by chitosan modified with glycidylmethacrylate and diethylenetriamine. Chem Eng J 171:159–166. doi:10.1016/j.cej.2011.03.081
Aliabadi M, Irani M, Ismaeili J, Piri H, Parnian MJ (2013) Electrospun nanofiber membrane of PEO/chitosan for the adsorption of nickel, cadmium, lead and copper ions from aqueous solution. Chem Eng J 220:237–243. doi:10.1016/j.cej.2013.01.021
Nguyen NT, Liu JH (2013) Fabrication and characterization of poly(vinyl alcohol)/chitosan hydrogel thin films via UV irradiation. Eur Polym J 49:4201–4211. doi:10.1016/j.eurpolymj.2013.09.032
Gad YH (2008) Preparation and characterization of poly(2-acrylamido-2-methylpropanesulfonic acid)/chitosan hydrogel using gamma irradiation and its application in wastewater treatment. Radiat Phys Chem 77:1101–1107. doi:10.1016/j.radphyschem.2008.05.002
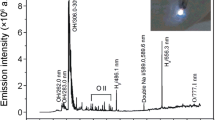
Malik MA, Ghaffar A, Malik SA (2001) Water purification by electrical discharges. Plasma Sources Sci Technol 10:82–91. doi:10.1088/0963-0252/10/1/311
Lu QF, Yu J, Gao JZ, Yang W, Li Y (2012) A promising absorbent of acrylic acid/poly(ethylene glycol) hydrogel prepared by glow-discharge electrolysis plasma. Cent Eur J Chem 10:1349–1359. doi:10.2478/s11532-012-0055-9
Lu QF, Yu J, Gao JZ, Yang W, Li Y (2011) Glow-discharge electrolysis plasma induced synthesis of polyvinylpyrrolidone/acrylic acid hydrogel and its adsorption properties for heavy-metal ions. Plasma Process Polym 8:803–814. doi:10.1002/ppap.201000144
Yu J, Yang GG, Pan YP, Lu QF, Yang W, Gao JZ (2014) Poly(acrylamide-co-acrylic acid) hydrogel induced by glow-discharge electrolysis plasma and its adsorption properties for cationic dyes. Plasma Sci Technol 16:767–776. doi:10.1088/1009-0630/16/8/07
Liu C, Bai R (2010) Extended study of DETA-functionalized PGMA adsorbent in the selective adsorption behaviors and mechanisms for heavy metal ions of Cu, Co, Ni, Zn, and Cd. J Colloid Interface Sci 350:282–289. doi:10.1016/j.jcis.2010.04.084
Yantasee W, Lin Y, Fryxell GE, Alford KL, Busche BJ, Johnson CD (2004) Selective removal of copper(II) from aqueous solutions using fine-grained activated carbon functionalized with amine. Ind Eng Chem Res 43:2759–2764. doi:10.1021/ie030182g
Koong LF, Lam KF, Barford J, McKay G (2013) A comparative study on selective adsorption of metal ions using aminated adsorbents. J Colloid Interface Sci 395:230–240. doi:10.1016/j.jcis.2012.12.047
Li Z, **ao D, Ge Y, Koehler S (2015) Surface-functionalized porous lignin for fast and efficient lead removal from aqueous solution. ACS Appl Mater Interfaces 7:15000–15009. doi:10.1021/acsami.5b03994
Irving H, Williams RJP (1953) The stability of transition-metal complexes. J Chem Soc 8:3192–3210. doi:10.1039/JR9530003192
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. doi:10.1021/ja00905a001
Pearson RG (1968) Hard and soft acids and bases, HSAB, part 1: fundamental principles. J Chem Educ 45:581–587. doi:10.1021/ed045p581
Şengil İA, Özacar M (2009) Competitive biosorption of Pb2+, Cu2+ and Zn2+ ions from aqueous solutions onto valonia tannin resin. J Hazard Mater 166:1488–1494. doi:10.1016/j.jhazmat.2008.12.071
Lam KF, Yeung KL, McKay G (2006) A rational approach in the design of selective mesoporous adsorbents. Langmuir 22:9632–9641. doi:10.1021/la061410p
Li K, Wang Y, Huang M, Yan H, Yang H, **ao S, Li A (2015) Preparation of chitosan-graft-polyacrylamide magnetic composite microspheres for enhanced selective removal of mercury ions from water. J Colloid Interface Sci 455:261–270. doi:10.1016/j.jcis.2015.05.043
Chen X, Lam KF, Mak SF, Yeung KL (2011) Precious metal recovery by selective adsorption using biosorbents. J Hazard Mater 186:902–910. doi:10.1016/j.jhazmat.2010.11.088
Beisebekov MM, Serikpayeva SB, Zhumagalieva SN, Beisebekov MK, Abilov ZA, Kosmella S, Koetz J (2015) Interactions of bentonite clay in composite gels of non-ionic polymers with cationic surfactants and heavy metal ions. Colloid Polym Sci 293:633–639. doi:10.1007/s00396-014-3463-x
Song X, Li C, Xu R, Wang K (2012) Molecular-ion-imprinted chitosan hydrogels for the selective adsorption of silver(I) in aqueous solution. Ind Eng Chem Res 51:11261–11265. doi:10.1021/ie3010989
Heidari A, Younesi H, Mehraban Z, Heikkinen H (2013) Selective adsorption of Pb(II), Cd(II), and Ni(II) ions from aqueous solution using chitosan-MAA nanoparticles. Int J Biol Macromol 61:251–263. doi:10.1016/j.ijbiomac
Li YH, Di Z, Ding J, Wu D, Luan Z, Zhu Y (2005) Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res 39:605–609. doi:10.1016/j.watres.2004.11.004
Tseng JY, Chang CY, Chang CF, Chen YH, Chang CC, Ji DR, Chiu CY, Chiang PC (2009) Kinetics and equilibrium of desorption removal of copper from magnetic polymer adsorbent. J Hazard Mater 171:370–377. doi:10.1016/j.jhazmat.2009.06.030
Joshi AA, Locke BR, Arce P, Finney WC (1995) Formation of hydroxyl radicals, hydrogen peroxide and aqueous electrons by pulsed streamer corona discharge in aqueous solution. J Hazard Mater 41:3–30. doi:10.1016/0304-3894(94)00099-3
Brisset JL, Moussa D, Doubla A, Hnatiuc E, Hnatiuc B, Youbi GK, Herry JM, Naïtali M, Bellon-Fontaine MN (2008) Chemical reactivity of discharges and temporal post-discharges in plasma treatment of aqueous media: examples of gliding discharge treated solutions. Ind Eng Chem Res 47:5761–5781. doi:10.1021/ie701759y
Joshi RP, Thagard SM (2013) Streamer-like electrical discharges in water: part II. Environmental applications. Plasma Chem Plasma Process 33:17–49. doi:10.1007/s11090-013-9436-x
Sun B, Sato M, Clements JS (1997) Optical study of active species produced by a pulsed streamer corona discharge in water. J Electrost 39:189–202. doi:10.1016/S0304-3886(97)00002-8
Baerdemaeker FD, Šimek M, Schmidt J, Leys C (2007) Characteristics of ac capillary discharge produced in electrically conductive water solution. Plasma Sources Sci Technol 16:341–354. doi:10.1088/0963-0252/16/2/018
Lukeš, P (2001) Water treatment by pulsed streamer corona discharge. PhD Thesis, Institute of Chemical Technology, Prague, Czech Republic
Jiang B, Zheng JT, Qiu S, Wu MB, Zhang QH, Yan ZF, Xue QZ (2014) Review on electrical discharge plasma technology for wastewater remediation. Chem Eng J 236:348–368. doi:10.1016/j.cej.2013.09.090
Neto CGT, Giacometti JA, Job AE, Ferreira FC, Fonseca JLC, Pereira MR (2005) Thermal analysis of chitosan based networks. Carbohydr Polym 62:97–103. doi:10.1016/j.carbpol.2005.02.022
Kyzas GZ, Siafaka PI, Pavlidou EG, Chrissafis KJ, Bikiaris DN (2015) Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem Eng J 259:438–448. doi:10.1016/j.cej.2014.08.019
Zeng G, Pang Y, Zeng Z, Tang L, Zhang Y, Liu Y, Zhang J, Lei X, Li Z, **ong Y, **e G (2012) Removal and recovery of Zn2+ and Pb2+ by imine-functionalized magnetic nanoparticles with tunable selectivity. Langmuir 28:468–473. doi:10.1021/la203810c
He J, Lu Y, Luo G (2014) Ca(II) imprinted chitosan microspheres: an effective and green adsorbent for the removal of Cu(II), Cd(II) and Pb(II) from aqueous solutions. Chem Eng J 244:202–208. doi:10.1016/j.cej.2014.01.096
Dragan ES, Cocarta AI, Dinu MV (2014) Facile fabrication of chitosan/poly(vinyl amine) composite beads with enhanced sorption of Cu2+. Equilibrium, kinetics, and thermodynamics. Chem Eng J 255:659–669. doi:10.1016/j.cej.2014.06.098
Lu Y, He J, Luo G (2013) An improved synthesis of chitosan bead for Pb(II) adsorption. Chem Eng J 226:271–278. doi:10.1016/j.cej.2013.04.078
**ao X, Hayashi F, Shiiba H, Selcuk S, Ishihara K, Namiki K, Shao L, Nishikiori H, Selloni A, Teshim K (2016) Platy KTiNbO5 as a selective Sr ion adsorbent: crystal growth, adsorption experiments, and DFT calculations. J Phys Chem C 120:11984–11992. doi:10.1021/acs.jpcc.6b02422
Chen CY, Chen SY (2004) Adsorption properties of a chelating resin containing hydroxy group and iminodiacetic acid for copper ions. J Appl Polym Sci 94:2123–2130. doi:10.1002/app.21079
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (No. 21367023, 21567025, and 11564037) and Natural Science Foundation of Gansu Province (No. 1308RJZA144), China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yu, J., Zheng, J., Lu, Q. et al. Selective adsorption and reusability behavior for Pb2+ and Cd2+ on chitosan/poly(ethylene glycol)/poly(acrylic acid) adsorbent prepared by glow-discharge electrolysis plasma. Colloid Polym Sci 294, 1585–1598 (2016). https://doi.org/10.1007/s00396-016-3920-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3920-9