Abstract
In this research paper, the approximate bound state solutions and thermodynamic properties of Schrӧdinger equation with modified exponential screened plus Yukawa potential (MESPYP) were obtained with the help Greene–Aldrich approximation to evaluate the centrifugal term. The Nikiforov–Uvarov (NU) method was used to obtain the analytical solutions. The numerical bound state solutions of five selected diatomic molecules, namely mercury hydride (HgH), zinc hydride (ZnH), cadmium hydride (CdH), hydrogen bromide (HBr) and hydrogen fluoride (HF) molecules were also obtained. We obtained the energy eigenvalues for these molecules using the resulting energy eigenequation and total unnormalized wave function expressed in terms of associated Jacobi polynomial. The resulting energy eigenequation was presented in a closed form and applied to study partition function (Z) and other thermodynamic properties of the system such as vibrational mean energy (U), vibrational specific heat capacity (C), vibrational entropy (S) and vibrational free energy (F). The numerical bound state solutions were obtained from the resulting energy eigenequation for some orbital angular quantum number. The results obtained from the thermodynamic properties are in excellent agreement with the existing literature. All numerical computations were carried out using spectroscopic constants of the selected diatomic molecules with the help of MATLAB 10.0 version. The numerical bound state solutions obtained increases with an increase in quantum state.
Similar content being viewed by others
Introduction
In quantum mechanics, the study of relativistic and nonrelativistic wave equations arouse the interest of different researchers [1,2,3,4,5]. Schrӧdinger equation is the nonrelativistic wave equation, while Dirac and Klein–Gordon equations are relativistic wave equation describing spin-half and spinless particles, respectively [6,7,8,9,10]. The total wave function provides implicitly the information about the quantum mechanical system. [11]. Researchers have adopted many methods in providing solutions to both relativistic and nonrelativistic wave equations. Among them are Nikiforov–Uvarov method [12,13,14,15,16], supersymmetric quantum mechanics approach [17], exact proper quantization [18] asymptotic iteration method [19], Wentzel–Kramers–Brillouin (WKB) approach [20] and many others [21,22,23,24,25,26,27,28,29]. These techniques have been used to solve some quantum mechanical potentials, like Hulthen, Yukawa, Poschl–Teller, Tietz–Wei, Tietz–Hua, exponential-type potentials, hyperbolic potentials, Kratzer potentials, screened-Kratzer potential, Mobius square, Hellmann, coulomb, Cornel, Killingbeck, Woods–Saxon, Deng-Fan, Hylleraas, Eckart, pseudoharmonic, Poschl–Teller, modified Yukawa potential and many others [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Yukawa potential which is otherwise known as screened Coulomb potential is a short-range potential with application in particle, high energy and molecular physics which is basically used for the description of interaction existing between atoms of diatomic molecules [49]. A lot of research work has been carried out on thermodynamic properties of some considerable potentials. Okon et.al [50] studied spin and pseudospin solutions of Dirac equation and its thermodynamic properties using hyperbolic Hulthen plus hyperbolic exponential inversely quadratic potential where they obtained numerical bound state solutions for both spin and pseudospin symmetries. Using nonrelativistic limit, they also obtain nonrelativistic energy eigenequation presented in a close form to study partition function and other thermodynamic properties. Okon et al. [51] studied thermodynamic properties and bound state solutions of Schrӧdinger equation using Mobius square plus screened-Kratzer potential within the framework of parametric Nikiforov–Uvarov (NU) method. In their study, they applied NU and semi-classical WKB to obtain bound state solutions and thermodynamic properties for two diatomic molecules (Carbon (II) oxide and Scandium Fluoride). Okorie et al. [52] studied energy spectra and thermodynamic properties of hyperbolic Poschl–Teller potential model. In that work, they solved Dirac equation using modified factorization method to obtain both relativistic and nonrelativistic ro-vibrational energy spectra and thermodynamic properties as applied to some diatomic molecules: Hydrogen chloride (HCl), chlorine (Cl2), carbon (II) oxide and lithium hydride (LiH). The potential they used is reduced to screened-Kratzer and Kratzer potential as special cases. Onate et al. [53] studied bound state solutions and thermal properties of modified Tietz–Hua potential using supersymmetric quantum mechanics approach where they study ro-vibrational energy spectra and thermodynamic properties as applicable to some diatomic properties. Purohit et al. [54] studied eigensolutions and various properties of the screened cosine Kratzer potential in D-dimension via relativistic and nonrelativistic treatment. Here, the screened-Kratzer potential model was extended to study rotational and vibrational energies for few heterogeneous diatomic molecules. They authors also extend the potential model to study the partition function as well as information theoretic measures like Tsallis, Renyi, Shannon and Fisher information entropies. Recently, researchers have studied potential model under the influence of Aharonov–Bohm flux and external magnetic field. Purohit et al. [55] studied the thermomagnetic properties of the screened-Kratzer potential under the influence of Aharonov–Bohm flux and external magnetic field where the obtained thermomagnetic properties as well as persistent current, magnetization and magnetic susceptibility. Okon et al. [56] investigated the effect of Aharonov–Bohm and external magnetic field on Hellmann plus screened-Kratzer potential where the energy equation was presented in a closed form and applied to study thermomagnetic properties as applicable to some diatomic molecules. Here, the authors obtained a normalized wave function expressed in terms of Jacobi polynomials as well as the wave function and probability density plots for some selected diatomic molecules. They also obtained other thermomagnetic properties including partition function, vibrational mean energy, vibrational heat capacity, magnetization, persistent current and magnetic susceptibility. Based on this motivation, in this paper, we study an approximate bound state solutions of Schrӧdinger equation and thermodynamic properties of newly proposed potential called modified exponential screened plus Yukawa potential (MESPYP) within the framework of parametric Nikiforov–Uvarov method. This is a new potential model that has not yet been studied to the best of our knowledge. This article is divided into seven sections. Section one gives the brief introduction of the article. Parametric Nikiforov–Uvarov method is presented in section two. The radial solution of the proposed potential is presented in section three. Numerical computation of energy eigenvalues is carried out in section four. Thermodynamic properties are presented in section five. Results and discussion are presented in section 6, while the article is concluded in section seven.
Review of parametric Nikiforov–Uvarov method
The NU method was proposed to solve the second-order linear differential equation by reducing it to a generalized equation of hypergeometric type of the form
where \(\sigma (s)\) and \(\sigma^{2} (s)\) are polynomials at most second degree, and \(\tilde{\tau }(s)\) is a first degree polynomial.
According to Tezcan and Sever [57], the parametric Nikiforov–Uvarov method is given as
The total wave function is given as
, while the total energy eigenequation is given as
where the parametric constants can be obtained as follows:
The Jacobi polynomial can be expressed in terms of Laguerre polynomial for a special case where \(C_{3} = 0\) [32]
and
Hence, the solution given by Eq. (3) becomes
Radial solution of Schrӧdinger equation with modified exponential screened plus Yukawa potential
The modified exponential screened plus Yukawa potential is given as
where \(D_{0} = \frac{{D_{e} }}{2} > 0\), \(D_{e}\) is the dissociation energy that describes the depth of the potential well, \(\alpha\) is the screening parameter which characterizes the strength of the potential. \(D_{1}\) is a real constant which also serve as a control parameter for the potential model, while \(r\) is the internuclear distance between the atoms of diatomic molecules.
The Schrödinger wave equation is given as:
Substituting the potential of Eq. (9) into Eq. (10) gives
where \(\lambda = l\left( {l + 1} \right)\).
Let us use Greene–Aldrich approximation to the centrifugal term as [24]
Substituting Eq. (12) into (11) gives
Using \(s = e^{ - 2\alpha r}\) , Eq. (13) can be transform from r to s-dimensions as
where \(\xi^{2} = - \frac{\mu E}{{2\alpha^{2} \hbar^{2} }}\begin{array}{*{20}c} , & {A = \frac{{\mu D_{0} }}{{2\alpha^{2} \hbar^{2} }}} \\ \end{array} \begin{array}{*{20}c} , & {B = \frac{{\mu D_{1} }}{{\alpha \hbar^{2} }}} \\ \end{array}\),
Comparing Eq. (14) with Eq. (2) gives
Using Eq. (6), other parametric constants can be obtained as
Using Eq. (5), the energy eigenequation for the proposed potential is given as
Using Eq. (4), the total unnormalized wave function is given as
Equation (18) can further be simplified as
where \(\chi_{1} = \sqrt {\frac{{\mu D_{0} }}{{2\alpha^{2} \hbar^{2} }} - \frac{{\mu E_{nl} }}{{2\alpha^{2} \hbar^{2} }}} \begin{array}{*{20}c} , & {\chi_{2} = } \\ \end{array} \frac{1}{2} + \sqrt {\frac{1}{4} + \lambda } .\)
Numerical computation of energy eigenvalues
Using Eq. (17), the numerical bound state solutions were carried out for fixed principal quantum number (n) with varying orbital angular quantum number \(l\) = 0, 1, 2 and 3.
Thermodynamic properties
In this section, we present the thermodynamic properties for the potential model. The thermodynamic properties of quantum systems can be obtained from the exact partition function given by [53, 54]
where \(\lambda\) is an upper bound of the vibrational quantum number obtained from the numerical solution of \(\frac{{{\text{d}}E_{n} }}{{{\text{d}}n}} = 0\), \(\beta = \frac{1}{kT}\) where \(k\) and T are Boltzmann constant and absolute temperature, respectively. In the classical limit, the summation in Eq. (19) can be replaced with an integral:
In order to obtain the partition function, energy equation (17) can be presented in a close and compact form as
The energy equation of (21) can be presented in a compact form as
where
Using Eq. (20), the partition function can be expressed as
Using Maple 10.0 version, the partition function of equation (25) can be evaluated as
Other thermodynamic properties can be obtained using the partition function.
-
(a)
Vibrational mean energy: The vibrational mean energy \(U(\beta )\) is
$$U(\beta ) = - \frac{\partial \ln Z(\beta )}{{\partial \beta }} \Rightarrow - \frac{1}{2}\left( {\frac{A + B + C + D}{{\sqrt \pi \beta \lambda \left( {e^{{2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) + e^{{ - 2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right)}}} \right),$$(27)where
$$\begin{aligned} & A = 8\sqrt \pi \beta \left( {2K_{2} K_{3} e^{{2\beta K_{2} K_{3} }} } \right)erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) - 2\sqrt \pi \beta \lambda K_{1} e^{{2\beta K_{2} K_{3} }} erf\left( {\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)} \right) \\ & B = - 2\sqrt \pi \beta \lambda K_{1} e^{{ - 2\beta K_{2} K_{3} }} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right)} \right) + 2\sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \lambda^{2} + 2\sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \\ & C = K_{3} + 2\sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( { - 4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \lambda^{2} - 2\sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( { - 4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3} - \sqrt \pi \lambda e^{{2\beta K_{2} K_{3} }} erf\left( {\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)} \right) \\ & D = - \sqrt \pi \lambda e^{{ - 2\beta K_{2} K_{3} }} erf\frac{{\left( {\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)} \right)}}{\lambda } \\ \end{aligned}$$(28) -
(b)
Vibrational specific heat capacity: The vibrational specific heat capacity \(C(\beta )\) is
$$\begin{aligned} C(\beta ) & = k\beta^{2} \left( {\frac{{\partial^{2} \ln Z(\beta )}}{{\partial \beta^{2} }}} \right) \\ & = \frac{1}{2}\left[ {K\left( {\frac{\begin{gathered} E + F + G + H + I + J + L + M + N + \hfill \\ O + P + Q + R + S + T + U + V + W + X \hfill \\ \end{gathered} }{{\left( {\sqrt { - \beta K_{2} } \lambda^{3} \pi \left[ {e^{{2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) + e^{{ - 2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right]^{2} } \right)}}} \right)} \right], \\ \end{aligned}$$(29)where
$$\begin{aligned} E & = 4K_{2} \lambda^{3} \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3} - 4K_{2} \lambda^{3} \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( { - 4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \\ & \quad + 2K_{2} \lambda \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \\ F & = K_{3}^{2} - 4K_{2} \lambda \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3}^{2} \\ & \quad + 2K_{2} \lambda \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( { - 4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3}^{2} \\ G & = \sqrt \pi \beta e^{{\frac{{\beta K_{2} \left( {6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3} K_{2} \lambda^{2} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right)} \right) \\ & \quad - \sqrt \pi \beta \,e^{{\frac{{2\beta K_{2} \left( { - 6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3} K_{2} \lambda^{2} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right) \\ H & = - 22\sqrt \pi \beta^{2} K_{2}^{2} K_{3} e^{{\frac{{\beta K_{2} \left( {\lambda^{2} + K_{3} } \right)^{2} }}{{\lambda^{2} }}}} \lambda^{4} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right) \\ & \quad - 22\sqrt \pi \beta^{2} K_{2}^{2} K_{3}^{2} e^{{\frac{{\beta K_{2} \left( {\lambda^{2} + K_{3} } \right)^{2} }}{{\lambda^{2} }}}} \lambda^{2} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right) \\ I & = 2\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{2\beta K_{2} \left( { - 6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3}^{3} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right) \\ & \quad + 22K_{2}^{2} \lambda^{4} \beta^{2} \sqrt \pi K_{3} e^{{\frac{{\beta K_{2} \left( {\lambda^{2} - K_{3} } \right)^{2} }}{{\lambda^{2} }}}} \,erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right) \\ J & = - 22K_{2}^{2} \lambda^{2} \beta^{2} \sqrt \pi K_{3}^{2} e^{{\frac{{\beta K_{2} \left( {\lambda^{2} + K_{3} } \right)^{2} }}{{\lambda^{2} }}}} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right)} \right) \\ \end{aligned}$$(30)$$\begin{aligned} L & = \sqrt \pi \beta e^{{\frac{{\beta K_{2} \left( {\lambda ^{2} + K_{3} } \right)^{2} }}{{\lambda ^{2} }}}} K_{3} K_{2} \lambda ^{2} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda ^{2} - K_{3} } \right)}}{\lambda }} \right)} \right) - \sqrt \pi \beta e^{{\frac{{\beta K_{2} \left( {\lambda ^{2} - K_{3} } \right)^{2} }}{{\lambda ^{2} }}}} K_{3} K_{2} \lambda ^{2} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda ^{2} + K_{3} } \right)}}{\lambda }} \right)} \right) \\ M & = 2\sqrt { - \beta K_{2} } \lambda ^{3} \pi erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda ^{2} + K_{3} } \right)}}{\lambda }} \right)} \right)erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( \begin{gathered} \lambda ^{2} - \hfill \\ K_{3} \hfill \\ \end{gathered} \right)}}{\lambda }} \right)} \right) \\ N & = 2\sqrt { - \beta K_{2} } \lambda ^{3} \pi erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda ^{2} + K_{3} } \right)}}{\lambda }} \right)} \right)erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( \begin{gathered} \lambda ^{2} - \hfill \\ K_{3} \hfill \\ \end{gathered} \right)}}{\lambda }} \right)} \right) + 2K_{2} \lambda ^{5} \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( {4K_{3} \lambda ^{2} + \lambda ^{4} + K_{3}^{2} } \right)}}{{\lambda ^{2} }}}} \\ \end{aligned}$$(31)$$\begin{aligned} O & = 4K_{2} \lambda^{5} \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( {\lambda^{4} + K_{3} } \right)}}{{\lambda^{2} }}}} + 2K_{2} \lambda^{5} \beta \sqrt { - \beta K_{2} } e^{{\frac{{2\beta K_{2} \left( { - 4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \\ & \quad + \sqrt { - \beta K_{2} } \lambda^{3} \pi e^{{4\beta K_{2} K_{3} }} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right)^{2} \\ Q & = \beta K_{2} \lambda^{4} \sqrt \pi e^{{\frac{{\beta K_{2} \left( {6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ P & = \sqrt { - \beta K_{2} } \lambda^{3} \pi e^{{4\beta K_{2} K_{3} }} erf\left( {\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)} \right)^{2} \\ S & = - 2\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{\beta K_{2} \left( {6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ & \quad + \sqrt { - \beta K_{2} } \lambda^{3} \pi e^{{ - 4\beta K_{2} K_{3} }} \\ T & = - 2\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{\beta K_{2} \left( { - 6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \lambda^{6} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ \end{aligned}$$(32)$$\begin{aligned} U & = - 6\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{\beta K_{2} \left( {6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \lambda^{2} K_{3}^{2} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ & \quad - 6\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{\beta K_{2} \left( {6K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \lambda^{4} K_{3} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ V & = 32\pi \beta^{2} K_{2}^{2} \lambda^{3} K_{3}^{2} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right)\sqrt { - \beta K_{2} } erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right) \\ & \quad + \beta K_{2} \lambda^{4} \sqrt \pi e^{{\frac{{\beta K_{2} \left( {\lambda^{2} - K_{3} } \right)^{2} }}{{\lambda^{2} }}}} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ W & = - 2\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{\beta K_{2} \left( {\lambda^{2} - K_{3} } \right)^{2} }}{{\lambda^{2} }}}} \lambda^{6} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ & \quad + 2\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{\beta K_{2} \left( {\lambda^{2} - K_{3} } \right)^{2} }}{{\lambda^{2} }}}} \lambda^{6} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ X & = 2\sqrt \pi \beta^{2} K_{2}^{2} e^{{\frac{{\beta K_{2} \left( {\lambda^{2} - K_{3} } \right)^{2} }}{{\lambda^{2} }}}} K_{3}^{2} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ \end{aligned}$$(33) -
(c)
Vibrational Entropy: The vibrational entropy is given as
$$\begin{aligned} S(\beta ) & = k\ln Z(\beta ) - k\beta \frac{\partial \ln Z(\beta )}{{\partial \beta }} \\ & = - \frac{1}{2}k\left[ {\frac{A + B + C + D + E}{{\sqrt \pi \beta \lambda e^{{2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) + e^{{ - 2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right)}}} \right], \\ \end{aligned}$$(34)where
$$\begin{aligned} A & = 4\ln 2 - \ln \pi - 2\ln \left( {e^{{ - \beta \left( { - 2K_{2} K_{3} + K_{1} } \right)}} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right)} \right) \\ & \quad + e^{{\left( { - 2\beta K_{2} K_{3} } \right)}} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right) \\ B & = 2\beta \left( { - \frac{1}{2}\left( { - 8\sqrt \pi \beta K_{2} \lambda K_{3} e^{{2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right)} \right)} \right) \\ & \quad + 2\sqrt \pi \beta \lambda K_{1} e^{{2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) \\ C & = 2\sqrt \pi \beta \lambda K_{1} e^{{ - 2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right) \\ & \quad - 2\sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \lambda^{2} - 2\sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3} \\ D & = - \sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( { - 4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} \lambda^{2} + 2\sqrt { - \beta K_{2} } e^{{\frac{{\beta K_{2} \left( {4K_{3} \lambda^{2} + \lambda^{4} + K_{3}^{2} } \right)}}{{\lambda^{2} }}}} K_{3} \\ E & = \sqrt \pi \lambda e^{{2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} + K_{3} } \right)}}{\lambda }} \right) + \sqrt \pi \lambda e^{{ - 2\beta K_{2} K_{3} }} erf\left( {\frac{{\sqrt { - \beta K_{2} } \left( {\lambda^{2} - K_{3} } \right)}}{\lambda }} \right) \\ \end{aligned}$$(35) -
(d)
Vibrational free energy: The vibrational free energy \(F(\beta )\) is given as
$$\begin{aligned} F(\beta ) & = - \frac{1}{\beta }\ln Z(\beta ) \\ & = - \frac{1}{\beta }\left[ {\ln \left( {\frac{1}{4}\frac{1}{{\sqrt { - \beta K_{2} } }}\left( \begin{gathered} e^{{\beta \left( {2K_{2} K_{3} - K_{1} } \right)}} \sqrt \pi \left( {e^{{2\beta K_{2} K_{3} }} erf\left( {\sqrt { - \beta K_{2} } \lambda + \frac{{K_{3} \sqrt { - \beta K_{2} } }}{\lambda }} \right)} \right) \hfill \\ + e^{{ - 2\beta K_{2} K_{3} }} erf\left( {\sqrt { - \beta K_{2} } \lambda - \frac{{K_{3} \sqrt { - \beta K_{2} } }}{\lambda }} \right) \hfill \\ \end{gathered} \right)} \right)} \right], \\ \end{aligned}$$(36)
Results and discussion
The numerical bound state energy for the selected diatomic molecules (HgH, ZnH, CdH, HBr and HF) has been computed. The spectroscopic constants of the five selected diatomic molecules used for the numerical computation are presented in Table 1.
Table 2 shows the numerical bound state solution of HgH with fixed screening parameter \(\alpha = 0.312\). The numerical table shows both monotonous increment and decrement with respect to quantum state for various orbital angular quantum number \(l\) = 0, 1, 2 and 3. The trend given in Tables 3, 4, 5 and 6 with screening parameters \(\alpha\) = 0.25, 0.218, 0.226 and 0.77 are spectroscopic constants for ZnH, CdH, HBr, and HF molecules, respectively, taken from Refs. [38, 48] for different values of the reduced mass (\(\mu\)) and dissociation energy (\(D_{e}\)). Throughout our computation, \(D_{1}\) is a control and optimizing parameter which is fixed to a value of 0.5, while \(\hbar\) takes a unitary value of 1.
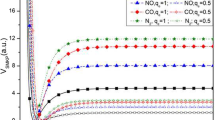
Figure 1a, b shows the variation of partition function (\(Z(\lambda ,\beta )\)) which increases exponentially with respect to \(\beta\) and decreases to -0.5 with respect to \(\lambda\). Figure 2a, b shows variation of vibrational mean energy (\(U(\lambda ,\beta )\)) with respect to \(\beta\) in Fig. 2a and variation with respect to \(\lambda\) as shown in Fig. 2b. In Fig. 2a, the vibrational mean energy increases with an increase in energy-dependent parameter but monotonically decreases with respect to \(\lambda\). The variation of vibrational specific heat capacity \(C(\lambda ,\beta )\) with respect to \(\beta\) is shown in Fig. 3a where there are several minimum turning points between \(\beta = 0.1\) and 0.2. In Fig. 3b, the vibrational specific heat capacity with respect to \(\lambda\) reproduces the same characteristics of vibrational mean energy with respect to \(\lambda\). In Fig. 4a, the vibrational entropy increases exponentially with respect to \(\beta\) but display an hyperbolic nature with respect \(\lambda\) as shown in Fig. 4b. The vibrational free energy decreases monotonically to a constant value of 0.08 with respect to \(\beta\) as shown in Fig. 5a but exhibits an hyperbolic nature with multiple maximum points at \(\lambda = - 1\) with respect to \(\lambda\) as shown in Fig. 5b. The trend of partition function and other thermodynamic properties obtained in this work is in excellent agreement with researches of refs [50,51,52,53,54] which affirm the accuracy of the work.
Conclusion
In this work, we study an approximate bound state solutions of Schrӧdinger wave equation with MESPYP potential model using parametric Nikiforov–Uvarov method where we obtain energy eigenequation and total unnormalized wave function expressed in terms of associated Jacobi polynomial. We obtained numerical bound state energies for five selected diatomic molecules. The resulting energy eigenequation was presented in a compact form and employed to study partition function and other thermodynamic properties. The numerical bound state solutions were carried out with a fixed control and optimizing parameter. The thermodynamic curves obtained are in excellent agreement to work of an existing literature.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
References
Okon, I.B., Popoola, O.O., Isonguyo, C.N., Antia, A.D.: Solutions of Schrӧdinger and Klein-Gordon equations with Hulthen plus inversely quadratic exponential Mie-Type potential. Phys. Sci. Int. J. 19(2), 1–27 (2018). https://doi.org/10.9734/PSIJ/2018/43610
Khokha, E.M., Abu-Shady, M., Abdel-Karim, T.A.: Quarkonium Masses in the N-dimensional space using the exact analytical iteration method. Int. J. Theor. Appl. Math. 2(2), 86–92 (2016). https://doi.org/10.11648/j.ijtam.20160202.19
Kuchin, S.M., Maksimenko, N.V.: Theoretical estimation of the spin-average mass spectra of heavy quarkonia and BC mesons. Univers. J. Phys. Appl. 1(3), 295–298 (2013). https://doi.org/10.1318/ujpa.2013.010310
Abu-Shady, M.: Heavy Quarkonia and bc mesons in the cornell potential with Harmonic oscillator potential in the N-dimensional Schrӧdinger equation. Int. J. Appl. Math. Theor. Phys. 2(2), 16–20 (2016). https://doi.org/10.11648/j.ijamtp.20160202.11
Al-Jamel, A., Widyan, H.: Heavy quarkonium mass spectra in a coulomb field plus inversely quadratic potential using Nikiforov–Uvarov method. Appl. Phys. Res. 4(3), 94–99 (2012). https://doi.org/10.5539/apr.v4n3p94
Antia, A.D., Eze, C.C., Okon, I.B., Akpabio, L.E.: Relativistic studies of Dirac equation with a spin-orbit coupled Hulthen potential including a Coulomb-like tensor interaction. J. Appl. Theor. Phys. Res. 3(2), 1–8 (2019). https://doi.org/10.24218/jatpr.2019.19
Okon, I.B., Popoola, O.O.: Bound state solution of Schrӧdinger equation with Hulthen plus generalised exponential coulomb potential using Nikiforov–Uvarov method. Int. J. Recent Adv. Phys. 4(3), 1–12 (2015). https://doi.org/10.14810/ijrap.2015.4301
Chalk, J.D.: A study of barrier penetration in quantum mechanics. Am. J. Phys. 56(29), 29–32 (1988). https://doi.org/10.1119/1.15425
Onate, C.A.: Approximate solutions of the nonrelativistic Schrӧdinger equation with an interaction of Coulomb and Hulthen potential. SOP Trans. Theor. Phys. 1(2), 118–127 (2014). https://doi.org/10.15764/TPHY.2014.02011
Omugbe, E., Osafile, O.E., Okon, I.B., Onyeaju, M.C.: Energy spectrum and the properties of Schoiberg potential using the WKB approximation approach. Mol. Phys. (2020). https://doi.org/10.1080/00268976.2020.1818860
Onate, C.A., Onyeaju, M.C., Abolarinwa, A., Lukman, A.F.: Analytical determination of theoretic quantities for multiple potential. Sci. Rep. 10, 17542 (2020). https://doi.org/10.1038/s41598-020-73372-x
Farout, M., Bassalat, A., Ikhdair, S.M.: Exact quantized momentum eigenvalues and eigenstates of a general potential model. J. Appl. Math. Phys. 8(7), 1434–1447 (2020)
Farout, M., Ikhdair, S.M.: Momentum eigensolutions of Feinberg-Horodecki potential equation with time dependent Screened Kratzer-Hellmann potential. J. Appl. Math. Phys. 8(07), 1207–2122 (2020)
Farout, M., Sever, R., Ikhdair, S.M.: Approximate solutions to the time dependent Kratzer plus screened—Coulomb potentials the Feinberg-Horodecki equation. Chin. Phys. B 29(6), 060303 (2020)
Farout, M., Bassalat, A., Ikhdair, S.M.: Feinberg-Horodecki exact momentum states of improved deformed exponential type potential. J. Appl. Math. Phys. 8, 1496–1506 (2020)
Farout, M., Yasin, M., Ikhdair, S.M.: Approximate bound state solutions for certain molecular potentials. J. Appl. Math. Phys. 9(4), 736–750 (2021)
Onate, C.A., Onyeaju, M.C., Okon, I.B., Adeoti, A.: Molecular energies of a modified and deformed—exponential type potential model. Chem. Phys. Impact 3, 10004 (2021). https://doi.org/10.1016/j.chphi.2021.100045
Serrano, F.A., Gu, X.-Y., Dong, S.H.: Qiang-Dong proper quantisation rule and its applications to exactly solvable quantum systems. J. Math. Phys. 51, 082103 (2010)
Falaye, B.J., Oyewumi, K.J., Ibrahim, T.T., Punyansena, M.A., Onate, C.A.: Bound state solutions of Manning Rosen potential. Can. J. Phys. 91, 98–104 (2013)
Omugbe, E., Osafile, O.E., Okon, I.B.: WKB energy expression for the radial Schrodinger equation with a generalised pseudoharmonic potential. Asian J. Phys. Chem. Sci. 8(2), 13–20 (2020). https://doi.org/10.9734/AJOPACS/2020/V8/230112
Qiang, W.C., Dong, S.H.: Arbitrary l—state solutions of the rotating Morse potential through the exact quantisation rule method. Phys. Lett. A 363(3), 169–176 (2007). https://doi.org/10.1016/j.physleta.2006.10.091
Pekeris, C.: The rotation-vibration coupling in diatomic molecules. Phys. Rev. 45, 98 (1934). https://doi.org/10.1103/PhysRev.45.98
Berkdemir, C., Han, J.: Any l-state solution of Morse potential through the Pekeris approximation and Nikiforov–Uvarov method. Chem. Phys. Lett. 409, 203–207 (2005). https://doi.org/10.1016/j.cplett.2005.05.021
Greene, R.L., Aldrich, C.: Variational wave functions for a screened Coulomb potential. Phys. Rev. A 14, 2363 (1976). https://doi.org/10.1103/PhysRevA.14.2363
Antia, A.D., Okon, I.B., Akankpo, A.O., Usanga, J.B.: Non-relativistic bound state solutions of modified quadratic—Yukawa plus q-deformed Eckart potential. J. Appl. Math. Phys. 8(4), 660–671 (2020). https://doi.org/10.4236/jamp.2020.84051
Antia, A.D., Okon, I.B., Umoren, E.B., Isonguyo, C.N.: Relativistic study of the spinless salpeter equation with a modified hylleraas potential. Ukranian J. Phys. 64(1), 27–33 (2019). https://doi.org/10.15407/ujpe64.1.27
Antia, A.D., Imeh, E.E., Umoren, E.U.: Approximate solutions of the nonrelativistic Schrӧdinger equation with inversely quadratic Yukawa plus Mobius square potential via parametic Nikiforov–Uvarov method. Adv. Phys. Theor. Appl. 44, 0638 (2015)
Ikot, A.N.: Solution to the Klien-Gordon equation with equal scalar and vector modified Hylleraas plus Expontial Rosen Morse potentials. Chin. Phys. Lett. 29, 060307 (2012). https://doi.org/10.1088/0256-307X/29/6/060307
Akpan, I.O., Antia, A.D., Ikot, A.N.: Bound-state solutions of the klein-Gordon equation with q-deformed equal scalar and vector Eckart potential using a newly improved approximation scheme. Int. Sch. Res. Notices 13(2012), article ID 79820 (2012)
Ikhdair, S.M.: An improved approximation scheme for the centrifugal term and the Hulthén potential. Eur. Phys. J. A 39(3), 307–314 (2009). https://doi.org/10.1140/epja/i2008-10715-2
Lucha, W., Schöberl, F.F.: Solving the Schrödinger equation for bound states with Mathematica 3.0. Int. J. Mod. Phys. C 10(4), 607–619 (1999). https://doi.org/10.1142/S0129183199000450
Okon, I.B., Isonguyo, C.N., Antia, A.D., Ikot, A.N., Popoola, O.O.: Fisher and Shannon information entropies for a noncentral inversely quadratic plus exponential Mie-type potential. Commun. Theor. Phys. 72, 065104 (2020). https://doi.org/10.1088/1572-9494/ab7ec9
Oyewumi, K.J., Sen, K.D.: Exact solutions of the Schrӧdinger equation for the pseudoharmonic potential: an application to some diatomic molecules. J. Math. Chem. 50, 1039–1059 (2012). https://doi.org/10.1007/s10910-011-9967-4
Jia, C.-S., Liu, J.-Y., Wang, P.-Q.: A new approximation scheme for the centrifugal term and the Hulthén potential. Phys. Lett. A 372(27), 4779–4782 (2008). https://doi.org/10.1016/j.physleta.2008.05.030
Stanek, J.: Approximate analytical solutions for arbitrary l—state of the Hulthén potential with an improved approximation of the centrifugal term. Cent. Eur. J. Chem. 9(4), 737–742 (2011). https://doi.org/10.2478/s11532-011-0050-6
Mbadjoun, B.T., Emaa, J.M., Yomi, J., Abiama, P.E., Ben-Bolie, G.H., Ateba, P.O.: Factorization method for exact solution of the non-central modified killingbeck potential plus a ring-shaped like potential. Mod. Phys. Lett. A 34, 1950072 (2019). https://doi.org/10.1142/S021773231950072X
Awoga, O.A., Ikot, A.N.: Approximate solution of Schrӧdinger equation in D dimension for inverted generalized hyperbolic potential. Pramana J. Phys. 79(3), 345–356 (2012). https://doi.org/10.1007/s12043-012-0328-z
Omugbe, E.: Non-relativistic energy spectrum of the Deng-Fan oscillator via the WKB approximation method. Asian J. Phys. Chem. Sci. 8(1), 26–36 (2020). https://doi.org/10.9734/ajopacs/2020/v8i130107
Omugbe, E., Osafile, O.E., Okon, I.B.: WKB energy expression for the radial Schrӧdinger equation with a generalized pseudoharmonic potential. Asian J. Phys. Chem. Sci. 8(2), 13–20 (2020). https://doi.org/10.9734/ajopacs/2020/v8i230112
Hassanabadi, H., Maghsodi, E., Zarrinkamar, S., Rahimov, H.: An approximate solutions of the Dirac equation for hyperbolic scalar and vector potentials and a coulomb tensor interaction by SUSYQM. Mod. Phys. Lett. A 26, 2703–2718 (2011). https://doi.org/10.1142/S0217732311037091
Gu, X.Y., Dong, S.H.: Energy spectrum of the Manning-Rosen potential including centrifugal term solved by exact and proper quantization rule. J. Math. Chem. 49, 2053 (2011). https://doi.org/10.1007/s10910-011-9877-5
Qiang, W.C., Dong, S.H.: The Manning–Rosen potential studied by new approximation scheme to the centrifugal term. Phys. Scr. 79, 045004 (2009). https://doi.org/10.1088/0031-8949/79/04/045004
Zhang-Qi, Ma., Cisneros, A.G., Xu, B.-W., Dong, S.H.: Energy spectrum of the trigonometric Rosen–Morse potential using improved quantisation rule. Phys. Lett. A 371, 180–184 (2007). https://doi.org/10.1016/j.physleta.2007.06.021
Parmar, R., Parmar, H.: Solution of the ultra generalized exponential-hyperbolic potential in multi-dimensional space. Few-Body Syst. 61, 1–28 (2020). https://doi.org/10.1007/s00601-020-01572-2
Chen, G.: Bound states for Dirac equation with Woods-Saxon potential. Acta Phys. Sin. 53(3), 680–683 (2004). https://doi.org/10.7498/aps.53.680
Isonguyo, C.N., Okon, I.B., Ikot, A.N., Hassanabadi, H.: Solution of Klein-Gordon equation for some diatomic molecules with new generalized Morse-like potential using SUSYQM. Bull. Korean Chem. Soc. 35(12), 3443–3446 (2014). https://doi.org/10.5012/bkcs.2014.35.12.3443
Okorie, U.S., Ibekwe, E.E., Ikot, A.N., Onyeaju, M.C., Chukwocha, E.O.: Thermal properties of the modified Yukawa potential. J. Korean Phys. Soc. 73(9), 1211–1218 (2019). https://doi.org/10.3938/jkps.73.1211
Onate, C.A.: Bound state solutions of the Schrӧdinger equation with second PӦschl-Teller like potential model and the vibrational partition function, mean energy and mean free energy. Chin. J. Phys. 54(2), 165–174 (2016). https://doi.org/10.1016/j.cjph.2016.04.001
Omugbe, E., Osafile, O.E., Onyeaju, M.C., Okon, I.B., Onate, C.A.: The unified treatment on the non-relativistic bound state solutions, thermodynamic properties and expectation values of exponential-type potentials. Can. J. Phys. (2020). https://doi.org/10.1139/cjp-2020-0368
Okon, I.B., Omugbe, E., Antia, A.D., Onate, C.A., Akpabio, L.E., Osafile, O.E.: Spin and pseudospin solutions and its thermodynamic properties using hyperbolic Hulthen plus hyperbolic exponential inversely quadratic potential. Sci. Rep. 11, 892 (2021). https://doi.org/10.1038/s41598-020-77756-x
Okon, I.B., Popoola, O.O., Omugbe, E., Antia, A.D., Isonguyo, C.N., Ituen, E.E.: Thermodynamic properties and bound state solutions of Schrӧdinger equation with Mobius Square plus Screened—Kratzer potential using Nikiforov–Uvarov method. Comput. Theor. Chem. 1196, 113132 (2021). https://doi.org/10.1016/j.comptc.2020.113132
Okorie, U.S., Ikot, A.N., Chukwuocha, E.O., Onyeaju, M.C., Amadi, P.O., Sithole, M.J., Rampho, G.J.: Energies spectra and thermodynamic properties of hyperbolic Poschl-Teller potential model. Int. J. Thermophys. 41, 91 (2020). https://doi.org/10.1007/s10765-020-02671-2
Onate, C.A., Onyeaju, M.C., Omugbe, E., Okon, I.B., Osafile, O.E.: Bound State Solutions and thermal properties of the modified Tietz-Hua potential. Sci. Rep. 11, 2129 (2021). https://doi.org/10.1038/s41598-021-81428-9
Purohit, K.R., Parmar, R.H., Rai, A.K.: Eigensolutions and various properties of the Screened Cosine Kratzer potential in D dimension via relativistic and nonrelativistic treatment. Eur. Phys. J. Plus 135, 286 (2020)
Purohit, K.R., Parmar, R.H., Rai, A.K.: Bound state solutions and thermodynamic properties of the screened kratzer cosine potential under the influence of magnetic and Aharanov-Bohm flux field. Ann. Phys. 424, 168335 (2021)
Okon, I.B., Onate, C.A., Omugbe, E., Okorie, U.S., Edet, C.O., Antia, A.D., Araujo, J.P., Isonguyo, C.N., Onyeaju, M.C., William, E.S., Horchani, R., Ikot, A.N.: Aharonov-Bohm (AB) flux and thermomagnetic properties of Hellmann plus Screened Kratzer potential as applied to diatomic molecules using Nikiforov–Uvarov Functional Analysis (NUFA) method. Mol. Phys. J. (2022). https://doi.org/10.1080/00268976.2022.2046295
Tezcan, C., Sever, R.: A general approach for the exact solution of the Schrödinger equation. Int. J. Theor. Phys. 48(2), 337–350 (2009). https://doi.org/10.1007/s10773-008-9806-y
Varshni, Y.P.: Comparative study of potential energy functions for diatomic molecules. Rev. Mod. Phys. 29, 664–682 (1957)
Awoga, O.A., Ikot, A.N., Akpan, I.O., Antia, A.D.: Solution of Schrӧdinger equation with exponential coshine-screened potential. Indian J. Pure Appl. Phys. 50, 217–223 (2012)
Acknowledgements
The authors are grateful to the editorial team of the journal as well as the reviewers for their positive comments and suggestions which we have used to improve the quality of this manuscript.
Funding
There is no funding in regard to the publication of this manuscript.
Author information
Authors and Affiliations
Contributions
ADA, IBO and CNI designed and wrote the main manuscript. AOA and NEE did the computations, and prepared the figures and tables. All authors reviewed the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antia, A.D., Okon, I.B., Isonguyo, C.N. et al. Bound state solutions and thermodynamic properties of modified exponential screened plus Yukawa potential. J Egypt Math Soc 30, 11 (2022). https://doi.org/10.1186/s42787-022-00145-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42787-022-00145-y