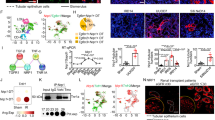
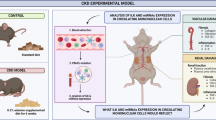
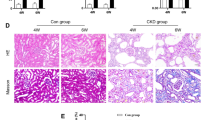
Abstract
Integrin αvβ6 holds promise as a therapeutic target for organ fibrosis, yet targeted therapies are hampered by concerns over inflammatory-related side effects. The role of αvβ6 in renal inflammation remains unknown, and clarifying this issue is crucial for αvβ6-targeted treatment of chronic kidney disease (CKD). Here, we revealed a remarkable positive correlation between overexpressed αvβ6 in proximal tubule cells (PTCs) and renal inflammation in CKD patients and mouse models. Notably, knockout of αvβ6 not only significantly alleviated renal fibrosis but also reduced inflammatory responses in mice, especially the infiltration of pro-inflammatory macrophages. Furthermore, conditional knockout of αvβ6 in PTCs in vivo and co-culture of PTCs with macrophages in vitro showed that depleting αvβ6 in PTCs suppressed the migration and pro-inflammatory differentiation of macrophages. Screening of macrophage activators showed that αvβ6 in PTCs activates macrophages via secreting IL-34. IL-34 produced by PTCs was significantly diminished by αvβ6 silencing, and reintroduction of IL-34 restored macrophage activities, while anti-IL-34 antibody restrained macrophage activities enhanced by αvβ6 overexpression. Moreover, RNA-sequencing of PTCs and verification experiments demonstrated that silencing αvβ6 in PTCs blocked hypoxia-stimulated IL-34 upregulation and secretion by inhibiting YAP expression, dephosphorylation, and nuclear translocation, which resulted in the activation of Hippo signaling. While application of a YAP agonist effectively recurred IL-34 production by PTCs, enhancing the subsequent macrophage migration and activation. Besides, reduced IL-34 expression and YAP activation were also observed in global or PTCs-specific αvβ6-deficient injured kidneys. Collectively, our research elucidates the pro-inflammatory function and YAP/IL-34/macrophage axis-mediated mechanism of αvβ6 in renal inflammation, providing a solid rationale for the use of αvβ6 inhibition to treat kidney inflammation and fibrosis.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a global public health threat with high morbidity and mortality, affecting about 9.1-13.4% of the general population and causing over a million deaths annually worldwide [1]. Renal fibrosis is a common and dynamic pathological process that drives nearly all types of kidney dysfunction to progress to CKD, eventually resulting in renal failure [2]. However, current therapies for CKD primarily address symptoms rather than directly ameliorating kidney fibrosis [3]. Therefore, it is urgent to develop safe and effective treatments to halt this life-threatening process.
Integrin αvβ6 is a member of the integrin family, a group of transmembrane receptors that play crucial roles in cell adhesion and communication between cells and their surrounding extracellular matrix. Previous research showed that integrin αvβ6 is up-regulated during multiple organ fibrosis, e.g., lung, liver, and kidney, and promotes fibrosis via activating the key profibrotic mediator, transforming growth factor-β1 (TGF-β1). This has positioned it as a promising therapeutic target for organ fibrosis [4,5,6,7,Aristolochic acids (AA) injection-induced nephropathy (AAN) model A model of renal fibrosis induced by AA injection as previously described [77]. Briefly, male WT and Itgb6-/- mice were intraperitoneally injected with 5 mg/kg AA (Sigma-Aldrich, A9451) or PBS every other day, and kidneys and serum were collected 10 days later for detection. Blood urea nitrogen (BUN) and serum creatinine levels were measured by commercial reagents and biochemical analyzers (Roche). For in vivo IL-34 administration, each Itgb6-/--uIRI mice were intraperitoneally injected with 1 μg of rmIL-34 (RD, 5195) on days 0 and 3 after uIRI. Control animals received PBS. For in vitro IL-34 treament, after hypoxia for 24 h, serial concentration gradients (0–250 ng/mL) of rmIL-34 (RD, 5195-ML) were added to the co-culture system of TKPTS cells and RAW264.7 cells, and the cells were treated for 12 h. Renal biopsy sections from CKD patients were subjected to antigen retrieval and non-specific binding sites were blocked with 5% BSA. According to the experimental requirements, kidney sections were incubated with sheep anti-human integrin β6 antibody (Ab) (PA5-47588, Thermo Fisher Scientific), mouse anti-human CD20 Ab (ab9475, Abcam), rabbit anti-human CD3 Ab (ab5690, Abcam), or mouse anti-human CD68 Ab (ab955, Abcam) at 4 °C overnight. Primary antibodies were labeled by incubating biotin-linked secondary antibodies, respectively. Mouse kidneys used for immunohistochemistry experiments were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm thick sections. After being blocked with 5% BSA, kidney sections were stained with rat anti-mouse F4/80 Ab (BIO-RAD, MCA497G), goat anti-mouse integrin β6 Ab (RD, AF2389), sheep anti-mouse IL-34 Ab (RD, AF5195), or rabbit anti-mouse YAP Ab (CST, 14074S) at 4 °C overnight. 3-3-diaminobenzidine (DAB) was used for color development in immunohistochemistry. The slides were then examined on a pathological section scanner (Kfbio, KF-PRO-020). Immunohistochemistry was quantified by counting the positive areas in 10 high-power fields (HPF). QRT-PCR was performed on mouse kidneys and cells. Trizol was used to lyse and extract total RNA from kidney homogenates, TKPTS cells, and RAW264.7 cells. The RNA extracted from trizol was extracted by chloroform, further precipitated in isopropanol, and washed with absolute ethanol. Finally, the RNA was dissolved in DEPC water. The concentration and quality of RNA were measured by NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific, USA). The RNA was reverse transcribed into cDNA according to a commercial reverse transcription kit (Vazyme, China). A PCR system was constructed using SYBR green dye, specific primers, and cDNA, and detection was performed in Applied Biosystems 7500 (Thermo Fisher Scientific, USA). Primer sequences are shown in Table S2. Mice were anesthetized with 1% pentobarbital and perfused with PBS until their kidneys became pale. The kidneys were mechanically cut into chunks and minced in RPMI 1640 containing 2% FBS at low temperatures before digestion. Digestion buffers were prepared with 1 mg/mL collagenase type II (Thermo Fisher Scientific, 17101015) and 0.5 mg/mL dispase type II (Thermo Fisher Scientific, 17105041) in RPMI 1640 containing 2% FBS. The kidneys were digested in a 200 rpm oscillator at 37 °C for 30 min. Post-digestion, the digestive fluid was filtered with a 70 μm filter and centrifuged. Red blood cells were lysed by 1 ml ACK Lysis Buffer (A1049201, Thermo Fisher Scientific). Centrifugation after the termination of fission was performed and the cell pellets were resuspended with PBS to obtain single-cell suspensions of mouse kidneys. Single-cell suspensions from mouse kidneys were prepared, and extracellular antigens were stained with flow cytometry antibodies. The antibodies used for Flow cytometry analysis are listed in Table S3. An AttuneNxT acoustic focusing cytometer (Thermo Fisher Scientific) was used for flow cytometry analysis, and FlowJo v.10 was used to process flow cytometry results. RIPA lysis buffer was used for protein extraction from mouse kidney homogenate, TKPTS cells, and RAW264.7 cells after supplementing protease inhibitors and phosphatase inhibitors. After centrifugation to remove structural proteins, the protein concentration was detected by the BCA method. Equal amounts of protein were separated by SDS-PAGE and electro-transferred to PVDF membranes. After blocking with 5% skim milk or 5% BSA, the PVDF membranes were incubated with primary antibodies overnight at 4 °C. The antibodies used in western blot were as follows: goat anti-mouse integrin β6 antibody (RD, AF2389), mouse anti-mouse α-SMA Ab (Sigma-Aldrich, A5228), rabbit anti-mouse Fibronectin Ab (BOSTER, BA1772), sheep anti-mouse IL-34 Ab (RD, AF5195), rabbit anti-mouse YAP Ab (CST, 14074 S), rabbit anti-mouse p-YAP (S127) Ab (CST, 4911 S), mouse anti-mouse GAPDH Ab (Abcam, ab8245), mouse anti-mouse α-Tubulin Ab (CST, 12351 S), and mouse anti-mouse β-actin Ab (Abcam, ab8226). After incubation was complete, unbound antibodies were washed with TBST (TBS: Tween, 1000:1). The horseradish peroxidase-conjugated secondary antibody derived from the primary antibody was incubated with the PVDF membrane for 1 h at room temperature, and the enhanced chemiluminescence (ECL) kit was used to develop specific protein bands. The image development of specific protein bands was quantitatively analyzed by ImageJ software. Kidney tissue was fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm thick kidney sections. Kidney sections were stained with Sirius red dye. The severity of tubulointerstitial fibrosis was assessed by a renal pathologist who was blinded to the experimental group, and the criterion was the area of Sirius red-positive area. Scoring was performed in 10 successive HPF fields in a blinded manner. Mouse kidney paraffin sections were permeabilized with 0.2% Triton X-100 after completion of antigen retrieval, and nonspecific sites were blocked with 10% donkey serum. LTL-fluorescein (Vector, FL-1321-2) was used to label proximal renal tubules, PNA-fluorescein (Vector, FL-1071-5) was used to label distal renal tubules, DBA-fluorescein (Vector, FL-1031-2) was used to label collecting ducts. Rabbit anti-mouse YAP Ab (CST, 14074 S), sheep anti-mouse IL-34 Ab (RD, AF5195), or rabbit anti-mouse KIM-1 Ab (Novus, NBP1-76701SS) was used to label the localization of YAP, IL-34, or KIM-1. The above primary antibodies were incubated overnight at 4 °C. After the unbound primary antibody was eluted with PBS, the corresponding FITC- or PE-labeled fluorescent secondary antibody was incubated for 1 h at room temperature. 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI) was used to label the cell nucleus. Confocal fluorescence microscopy (ZEISS, LSM880 with Airyscan) was used to capture fluorescent signals, and ImageJ software was used to perform quantitative statistics on the co-localization of fluorescent signals. In order to simulate the ischemia-reperfusion injury model in vivo, we used an in vitro H/R injury cell model. The resume of the H/R injury cell model was performed as described previously [Quantification and statistical analysis GraphPad Prism v9.0 software was used for data statistics and visual presentation. Experimental data were presented as mean ± SEM. An unpaired Student’s t-test was used to compare the two groups. In more than two groups of comparison using general one-way ANOVA for statistics. All experiments were performed in at least three biologically independent replicates. The p-value < 0.05 indicated a statistically significant difference. The statistical significance was respectively expressed as: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; #p < 0.05; ##p < 0.01; ###p < 0.001; ####p < 0.0001; ns, not significant.In vivo and in vitro treatment of recombinant mouse IL-34 (rmIL-34)
Immunohistochemical staining
Quantitative real-time PCR (qRT-PCR)
Preparation of kidney single-cell suspension
Flow cytometry
Western blot
Collagen fiber detection
Immunofluorescence
Hypoxia/reoxygenation (H/R) injury cell model
Data availability
The Raw and processed transcription sequencing data of TKPTS have been deposited at the GEO with the project number: GSE253494. All other study data are included in the article and/or the supplement. Any additional data in this work are available from the corresponding authors upon request.
References
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–33.
Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. Chronic kidney disease. Nat Rev Dis Prim. 2017;3:17088.
Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16:269–88.
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28.
Locatelli L, Cadamuro M, Spirlì C, Fiorotto R, Lecchi S, Morell CM, et al. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology. 2016;63:965–82.
Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, et al. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol. 2003;163:1261–73.
Zhang Z, Wang Z, Liu T, Tang J, Liu Y, Gou T. et al. Exploring the role of ITGB6: fibrosis, cancer, and other diseases. Apoptosis. 2023;29:570–85.
Pang X, He X, Qiu Z, Zhang H, **e R, Liu Z, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8:1.
Slack RJ, Macdonald SJF, Roper JA, Jenkins RG, Hatley RJD. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov. 2022;21:60–78.
Raghu G, Mouded M, Chambers DC, Martinez FJ, Richeldi L, Lancaster LH, et al. A phase IIb randomized clinical study of an anti-α(v)β(6) monoclonal antibody in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2022;206:1128–39.
Liu J, Nair V, Zhao YY, Chang DY, Limonte C, Bansal N, et al. Multi-scalar data integration links glomerular angiopoietin-tie signaling pathway activation with progression of diabetic kidney disease. Diabetes. 2022;71:2664–76.
Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157–62.
Annuk M, Zilmer M, Lind L, Linde T, Fellström B. Oxidative stress and endothelial function in chronic renal failure. J Am Soc Nephrol. 2001;12:2747–52.
Izquierdo MC, Martin-Cleary C, Fernandez-Fernandez B, Elewa U, Sanchez-Niño MD, Carrero JJ, et al. CXCL16 in kidney and cardiovascular injury. Cytokine Growth Factor Rev. 2014;25:317–25.
Romejko K, Markowska M, Niemczyk S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int J Mol Sci. 2023;24:10470.
Xu L, Sharkey D, Cantley LG. Tubular GM-CSF Promotes Late MCP-1/CCR2-Mediated Fibrosis and Inflammation after Ischemia/Reperfusion Injury. J Am Soc Nephrol. 2019;30:1825–40.
Doke T, Abedini A, Aldridge DL, Yang YW, Park J, Hernandez CM, et al. Single-cell analysis identifies the interaction of altered renal tubules with basophils orchestrating kidney fibrosis. Nat Immunol. 2022;23:947–59.
Cormican S, Negi N, Naicker SD, Islam MN, Fazekas B, Power R, et al. Chronic Kidney Disease Is Characterized by Expansion of a Distinct Proinflammatory Intermediate Monocyte Subtype and by Increased Monocyte Adhesion to Endothelial Cells. J Am Soc Nephrol. 2023;34:793–808.
Hoeft K, Schaefer GJL, Kim H, Schumacher D, Bleckwehl T, Long Q, et al. Platelet-instructed SPP1(+) macrophages drive myofibroblast activation in fibrosis in a CXCL4-dependent manner. Cell Rep. 2023;42:112131.
Kim YG, Kim EY, Ihm CG, Lee TW, Lee SH, Jeong KH, et al. Gene polymorphisms of interleukin-17 and interleukin-17 receptor are associated with end-stage kidney disease. Am J Nephrol. 2012;36:472–7.
González-Guerrero C, Morgado-Pascual JL, Cannata-Ortiz P, Ramos-Barron MA, Gómez-Alamillo C, Arias M, et al. CCL20 blockade increases the severity of nephrotoxic folic acid-induced acute kidney injury. J Pathol. 2018;246:191–204.
Steele H, Cheng J, Willicut A, Dell G, Breckenridge J, Culberson E, et al. TNF superfamily control of tissue remodeling and fibrosis. Front Immunol. 2023;14:1219907.
Cormican S, Griffin MD. Fractalkine (CX3CL1) and its receptor CX3CR1: A promising therapeutic target in chronic kidney disease? Front Immunol. 2021;12:664202.
Lemos DR, McMurdo M, Karaca G, Wilflingseder J, Leaf IA, Gupta N, et al. Interleukin-1β Activates a MYC-Dependent Metabolic Switch in Kidney Stromal Cells Necessary for Progressive Tubulointerstitial Fibrosis. J Am Soc Nephrol. 2018;29:1690–705.
Wang S, Diao H, Guan Q, Cruikshank WW, Delovitch TL, Jevnikar AM, et al. Decreased renal ischemia-reperfusion injury by IL-16 inactivation. Kidney Int. 2008;73:318–26.
Xu S, Yang X, Chen Q, Liu Z, Chen Y, Yao X, et al. Leukemia inhibitory factor is a therapeutic target for renal interstitial fibrosis. EBioMedicine. 2022;86:104312.
Lee J, Lee Y, Kim KH, Kim DK, Joo KW, Shin SJ, et al. Chemokine (C-C Motif) Ligand 8 and Tubulo-Interstitial Injury in Chronic Kidney Disease. Cells. 2022;11.
Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15:144–58.
Distler JHW, Györfi AH, Ramanujam M, Whitfield ML, Königshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15:705–30.
Cao C, Yao Y, Zeng R. Lymphocytes: Versatile Participants in Acute Kidney Injury and Progression to Chronic Kidney Disease. Front Physiol. 2021;12:729084.
Lever JM, Hull TD, Boddu R, Pepin ME, Black LM, Adedoyin OO, et al. Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight. 2019;4.
**g C, Castro-Dopico T, Richoz N, Tuong ZK, Ferdinand JR, Lok LSC, et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc Natl Acad Sci USA. 2020;117:15160–71.
Clements M, Gershenovich M, Chaber C, Campos-Rivera J, Du P, Zhang M, et al. Differential Ly6C Expression after Renal Ischemia-Reperfusion Identifies Unique Macrophage Populations. J Am Soc Nephrol. 2016;27:159–70.
Rudman-Melnick V, Adam M, Potter A, Chokshi SM, Ma Q, Drake KA, et al. Single-Cell Profiling of AKI in a Murine Model Reveals Novel Transcriptional Signatures, Profibrotic Phenotype, and Epithelial-to-Stromal Crosstalk. J Am Soc Nephrol. 2020;31:2793–814.
Koivisto L, Bi J, Häkkinen L, Larjava H. Integrin αvβ6: Structure, function and role in health and disease. Int J Biochem Cell Biol. 2018;99:186–96.
Shimodaira T, Matsuda K, Uchibori T, Sugano M, Uehara T, Honda T. Upregulation of osteopontin expression via the interaction of macrophages and fibroblasts under IL-1b stimulation. Cytokine. 2018;110:63–9.
Jung YJ, Lee AS, Nguyen-Thanh T, Kim D, Kang KP, Lee S, et al. SIRT2 Regulates LPS-Induced Renal Tubular CXCL2 and CCL2 Expression. J Am Soc Nephrol. 2015;26:1549–60.
Qu C, Edwards EW, Tacke F, Angeli V, Llodrá J, Sanchez-Schmitz G, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–41.
Wang L, Li S, Luo H, Lu Q, Yu S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J Exp Clin Cancer Res. 2022;41:303.
Jiang W, Zhang Y, Sheng Y, Liu M, Du C, Pan X, et al. Overexpression of IFIT1 protects against LPS-induced acute lung injury via regulating CCL5-p65NF-κB signaling. Int Immunopharmacol. 2023;114:109485.
Woltman AM, de Fijter JW, van der Kooij SW, Jie KE, Massacrier C, Caux C, et al. MIP-3alpha/CCL20 in renal transplantation and its possible involvement as dendritic cell chemoattractant in allograft rejection. Am J Transpl. 2005;5:2114–25.
Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–62.
Hirani D, Alvira CM, Danopoulos S, Milla C, Donato M, Tian L. et al. Macrophage-derived IL-6 trans-signalling as a novel target in the pathogenesis of bronchopulmonary dysplasia. Eur Respir J. 2022;59:2002248.
Pawluczyk IZA, Soares MSF, Barratt WA, Brown JR, Bhachu JS, Selvaskandan H, et al. Macrophage interactions with collecting duct epithelial cells are capable of driving tubulointerstitial inflammation and fibrosis in immunoglobulin A nephropathy. Nephrol Dial Transpl. 2020;35:1865–77.
Wen Y, Lu X, Ren J, Privratsky JR, Yang B, Rudemiller NP, et al. KLF4 in Macrophages Attenuates TNFα-Mediated Kidney Injury and Fibrosis. J Am Soc Nephrol. 2019;30:1925–38.
Baek JH, Zeng R, Weinmann-Menke J, Valerius MT, Wada Y, Ajay AK, et al. IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest. 2015;125:3198–214.
Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Ren Physiol. 2015;308:F69–75.
Lin W, Xu D, Austin CD, Caplazi P, Senger K, Sun Y, et al. Function of CSF1 and IL34 in Macrophage Homeostasis, Inflammation, and Cancer. Front Immunol. 2019;10:2019.
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL, et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J Am Soc Nephrol. 2018;29:919–35.
Uyangaa E, Kim JH, Patil AM, Choi JY, Kim SB, Eo SK. Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6Chi Monocytes and NK Cells via CCL2-CCL3 Cascade. PLoS Pathog. 2015;11:e1005256.
Gschwend J, Sherman SPM, Ridder F, Feng X, Liang HE, Locksley RM. et al. Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth. J Exp Med. 2021;218:e20210745.
Ni B, Zhang D, Zhou H, Zheng M, Wang Z, Tao J, et al. IL-34 attenuates acute T cell-mediated rejection following renal transplantation by upregulating M2 macrophages polarization. Heliyon. 2024;10:e24028.
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer Cell. 2017;31:127–41.
Wang Z, Wang F, Ding XY, Li TE, Wang HY, Gao YH, et al. Hippo/YAP signaling choreographs the tumor immune microenvironment to promote triple negative breast cancer progression via TAZ/IL-34 axis. Cancer Lett. 2022;527:174–90.
Trevillian P, Paul H, Millar E, Hibberd A, Agrez MV. alpha(v)beta(6) Integrin expression in diseased and transplanted kidneys. Kidney Int. 2004;66:1423–33.
Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–8.
Hogmalm A, Sheppard D, Lappalainen U, Bry K. beta6 Integrin subunit deficiency alleviates lung injury in a mouse model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2010;43:88–98.
Kurbet AS, Hegde S, Bhattacharjee O, Marepally S, Vemula PK, Raghavan S. Sterile Inflammation Enhances ECM Degradation in Integrin β1 KO Embryonic Skin. Cell Rep. 2016;16:3334–47.
Chen H, Chen L, Wang X, Ge X, Sun L, Wang Z, et al. Transgenic overexpression of ITGB6 in intestinal epithelial cells exacerbates dextran sulfate sodium-induced colitis in mice. J Cell Mol Med. 2021;25:2679–90.
Roy-Chaudhury P, Hillis G, McDonald S, Simpson JG, Power DA. Importance of the tubulointerstitium in human glomerulonephritis. II. Distribution of integrin chains beta 1, alpha 1 to 6 and alpha V. Kidney Int. 1997;52:103–10.
Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, et al. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol. 2007;170:110–25.
Chung S, Overstreet JM, Li Y, Wang Y, Niu A, Wang S. et al. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight. 2018;3:e123563.
Ying WZ, Li X, Rangarajan S, Feng W, Curtis LM, Sanders PW. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest. 2019;129:2792–806.
Qi R, Yang C. Renal tubular epithelial cells: the neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018;9:1126.
Zheng Z, Li C, Shao G, Li J, Xu K, Zhao Z, et al. Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death Dis. 2021;12:754.
Habshi T, Shelke V, Kale A, Lech M, Gaikwad AB. Hippo signaling in acute kidney injury to chronic kidney disease transition: Current understandings and future targets. Drug Discov Today. 2023;28:103649.
Pearson JD, Huang K, Pacal M, McCurdy SR, Lu S, Aubry A, et al. Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity. Cancer Cell. 2021;39:1115–34.e12.
Cosset É, Ilmjärv S, Dutoit V, Elliott K, von Schalscha T, Camargo MF, et al. Glut3 Addiction Is a Druggable Vulnerability for a Molecularly Defined Subpopulation of Glioblastoma. Cancer Cell. 2017;32:856–68.e5.
Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, Dobie R, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat Commun. 2016;7:12502.
Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen D, et al. Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep. 2020;30:793–806.e6.
Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–48.
Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20:888–99.
Jang M, An J, Oh SW, Lim JY, Kim J, Choi JK, et al. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat Biomed Eng. 2021;5:114–23.
Kaukonen R, Mai A, Georgiadou M, Saari M, De Franceschi N, Betz T, et al. Normal stroma suppresses cancer cell proliferation via mechanosensitive regulation of JMJD1a-mediated transcription. Nat Commun. 2016;7:12237.
Zhu H, Liao J, Zhou X, Hong X, Song D, Hou FF, et al. Tenascin-C promotes acute kidney injury to chronic kidney disease progression by impairing tubular integrity via αvβ6 integrin signaling. Kidney Int. 2020;97:1017–31.
Peng Y, Li L, Shang J, Zhu H, Liao J, Hong X, et al. Macrophage promotes fibroblast activation and kidney fibrosis by assembling a vitronectin-enriched microenvironment. Theranostics. 2023;13:3897–913.
Jadot I, Declèves AE, Nortier J, Caron N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. Int J Mol Sci. 2017;18:297.
Li J, Sun X, Yang N, Ni J, **e H, Guo H, et al. Phosphoglycerate mutase 5 initiates inflammation in acute kidney injury by triggering mitochondrial DNA release by dephosphorylating the pro-apoptotic protein Bax. Kidney Int. 2023;103:115–33.
Sen P, Helmke A, Liao CM, Sörensen-Zender I, Rong S, Bräsen JH, et al. SerpinB2 Regulates Immune Response in Kidney Injury and Aging. J Am Soc Nephrol. 2020;31:983–95.
Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S, et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med. 2016;8:352ra108.
Acknowledgements
We are grateful to Guangzhou Genedenovo Biotechnology Co., Ltd for assisting in sequencing and/or bioinformatics analysis. We thank the authors of the GSE180394 and GSE139506 datasets for their data sharing.
Funding
This work was supported by First affiliated hospital of Sun Yat-sen University (SYSU-FAH) in Guangzhou, the National Natural Science Foundation of China 82270764, 82022009 to Y.Z., the Guangdong Natural Science Fund 2017A030306013 to Y.Z., Guangdong Special Support Program 2017TQ04R549 to Y.Z., the Medical Scientific Research Foundation of Guangdong Province of China A2024182 to Z.L., NHC Key Laboratory of Clinical Nephrology (Sun Yat-Sen University) and Guangdong Provincial Key Laboratory of Nephrology 2020B1212060028.
Author information
Authors and Affiliations
Contributions
CZ, RZ, and ZL designed, performed and interpreted all experiments. CZ and RZ carried out data analysis. CZ, RZ, ZL, XHan, ZT, FL, XHu, RL, JS, QP, and RW performed all animal work. ZP and GW provided Itgb6 knock-out mice. CZ, ZL, FL, and RL collected human kidney biopsies. CZ and RZ wrote the original manuscript, and ZL, WC, and YZ revised and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All human participants in this study signed informed consent and were approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University (approval numbers 2022(602), 2016(215)). All animal experiments in this study were approved by the Animal Ethics Committee of Sun Yat-sen University of Medical Sciences.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Hans-Uwe Simon
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, C., Zheng, R., Han, X. et al. Knockout of integrin αvβ6 protects against renal inflammation in chronic kidney disease by reduction of pro-inflammatory macrophages. Cell Death Dis 15, 397 (2024). https://doi.org/10.1038/s41419-024-06785-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06785-5
- Springer Nature Limited