Abstract
Lead (Pb) is a known nephrotoxicant that causes damage to proximal tubular cells. Autophagy has an important protective role in various renal injuries, but the role of autophagy in Pb-elicited nephrotoxicity remains largely unknown. In this study, Pb promoted the accumulation of autophagosomes in primary rat proximal tubular (rPT) cells, and subsequent findings revealed that this autophagosome accumulation was caused by the inhibition of autophagic flux. Moreover, Pb exposure did not affect the autophagosome–lysosome fusion in rPT cells. Next, we found that Pb caused lysosomal alkalinization, may be through suppression of two V-ATPase subunits. Simultaneously, Pb inhibited lysosomal degradation capacity by affecting the maturation of cathepsin B (CTSB) and cathepsin D (CTSD). Furthermore, translocation of CTSB and CTSD from lysosome to cytoplasm was observed in this study, suggesting that lysosomal membrane permeabilization (LMP) occurred in Pb-exposed rPT cells. Meanwhile, Pb-induced caspase-3 activation and apoptosis were significantly but not completely inhibited by CTSB inhibitor (CA 074) and CTSD inhibitor (pepstatin A), respectively, demonstrating that LMP-induced lysosomal enzyme release was involved in Pb-induced apoptosis in rPT cells. In conclusion, Pb-mediated autophagy blockade in rPT cells is attributed to the impairment of lysosomal function. Both inhibition of autophagic flux and LMP-mediated apoptosis contribute to Pb-induced nephrotoxicity in rPT cells.
Similar content being viewed by others
Main
Lead (Pb) is one of the most abundant toxic heavy metals in the environment which causes a broad range of biochemical, physiological and behavioral dysfunctions in human beings.1 Globally, Pb exposure is ubiquitous and routes of Pb exposure include inhalation of Pb-contaminated dust particles or aerosols, and ingestion of Pb-contaminated food or water.1, 2 The persistence of Pb in humans and its associated health risk is a matter of serious concern and a global issue. Pb is also a cumulative toxicant that is stored in the body for a long term, which exerts potent toxic effects on different tissues.3 Kidney is one of the most sensitive target organs for Pb toxicity, while the proximal tubule is the major site of Pb-induced renal injury.4 However, proximal tubular cells were less applied in the studies of Pb-induced nephrotoxicity. Primary cultures can better represent the live tissue than permanent cell lines, which are ideal for in vitro toxicity studies. Thus, primary rPT cells were established to elucidate low-level Pb-induced nephrotoxicity in this study.
Autophagy is a highly dynamic multi-step biological process which maintains cellular homeostasis via the degradation and recycling of damaged organelles, misfolded proteins, and long-lived macromolecules in lysosomes.5 Previous studies have demonstrated that basal autophagy is vital for normal proximal tubule function, and genetic or pharmacologic blockade of autophagy strongly enhanced acute kidney injury induced by cisplatin or ischemia-reperfusion.6, 7, 8, 9 Moreover, report by Lv et al.10 and Sui et al.11 showed that Pb promoted the autophagy in cultured osteoblasts and cardiofibroblasts, respectively. By contrast, our research group has recently found that 0.5 μM Pb treatment for 12 h blocked the autophagic flux in rPT cells,12 while the underlying molecular mechanism of impaired autophagic flux during Pb exposure remains to be elucidated.
Lysosomes are acidic organelles that contain various hydrolytic enzymes, which serve as cellular recycling centers for cargos received mainly from autophagy and endocytosis. Normal lysosomal degradation function is crucial to maintain cellular homeostasis and enable cell survival in the physiological state.13, 14 Due to its high hydrolase content, lysosomes are potentially harmful to the cell when damage occurs to the lysosomal membrane to induce lysosomal membrane permeabilization (LMP).14 LMP has been shown to cause the release of cathepsins and other hydrolases from the lysosomal lumen to the cytosol, which is a critical step in lysosome-mediated apoptosis.13 We have previously demonstrated that the apoptotic death was the chief mechanism in low-dose (0-1.0 μM) Pb-induced nephrotoxicity in rPT cells,15 which enable us to think whether LMP is involved in Pb-induced apoptosis in rPT cells. Based on the previous studies, this study will offer further evidences to elucidate the possible interaction mechanism between autophagy inhibition, impairment of lysosomal function and apoptosis in Pb-exposed rPT cells.
Results
Enhanced expression of autophagic marker LC3-II in Pb-exposed rPT cells
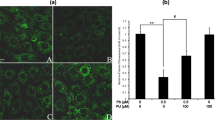
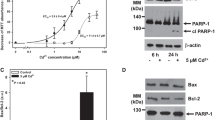
Immunoblot analysis of endogenous LC3-II has been widely used to reflect the progression of autophagy. Firstly, protein levels of LC3-II in rPT cells treated with Pb (0.25, 0.5 and 1 μM) for 3 h (Figure 1a), 6 h (Figure 1b) and 12 h (Figure 1c) was detected to investigate the effect of Pb exposure on autophagy, respectively. After 3 h treatment, only 1 μM Pb significantly elevated the LC3-II protein levels (Figure 1a). Significant differences were observed in the LC3-II protein levels after exposed to Pb (0.25, 0.5 and 1 μM) for 6 h (Figure 1b) and 12 h (Figure 1c), respectively; but 0.5 μM Pb resulted in a somewhat greater increase in LC3-II level than 1 μM Pb at these two time points. Likewise, there was a time-dependent enhancement of LC3-II protein level in 0.5 μM Pb-treated cells (Figure 1d). Thus, 0.5 μM Pb and 12 h exposure time were selected in subsequent experiments.
Expression of the autophagy marker protein LC3-II in Pb-exposed rPT cells. Cells were incubated with increasing doses of Pb for 3 h (a), 6 h (b) and 12 h (c) to assess the protein levels of LC3-II. (d) Cells were treated with 0.5 μM Pb for different time periods to determine the protein levels of LC3-II. Upper panels: representative western blot images; lower panels: quantitative analysis of protein levels (mean±S.E.M., n=4). ns, not significant, *P<0.05, **P<0.01, as compared with control
Autophagic flux was impaired by Pb treatment in rPT cells
Although the amount of LC3-II correlates with the number of autophagosomes, increased numbers of autophagosomes can be associated either with increased autophagosomes synthesis or decreased autophagosomes turnover.16 To distinguish these two possibilities, we assessed the autophagic flux. First, the effect of Pb exposure on the autophagosome formation in the presence of 3-MA or CQ (two well-defined autophagy inhibitors) was assessed using transient transfection method (Figures 2a and b). Co-incubation of Pb with 3-MA, which blocks the upstream steps of autophagy,20 It is also a concentration dependent meta-chromatic fluorescent dye. Upon the excitation by blue light, AO can be visualized as red fluorescence at high concentrations (in intact lysosomes) and green fluorescence at low concentrations (in the cytosol and the nucleus). Thus, AO relocation, from lysosomes to cytosol, and the decrease of granular (lysosomal) red fluorescence (dimer form) in combination with the increased diffuse (cytosolic) green fluorescence (monomer form) may imply the deterioration of lysosomal membrane stability with a decreased proton gradient, which permits the leakage of the lysosomal contents to cytosol.20 After incubated with 0.5 μM Pb (12 h) or 50 μM CQ (3 h) or EBSS medium (2 h), respectively, cells grown on coverslips in 24-well plates were loaded with 5 μg/ml AO at 37 °C for 30 min, rinsed twice with warm (37 °C) PBS and examined under confocal laser scanning microscope (TCS SPE, Leica, Germany) with excitation at 488 nm. Green fluorescence (emission peak between 530 and 550 nm) and red fluorescence (emission peak at about 650 nm) were simultaneously collected by two separate windows.
Lyso-Tracker Red staining
Cells were seeded on sterile coverslips placed in 24-well plates. After treatment with 0.5 μM Pb (12 h), EBSS medium (2 h) or 50 μM CQ (3 h), respectively, cells were incubated with 100 nM LTR (diluted in DMEM-F12 medium) for 30 min under ideal growth conditions (37 °C, 5% CO2) to label the lysosomes. Then slides were rapidly washed with warm PBS (37 °C) for three times, mounted as described above and observed under a laser scanning confocal microscope (TCS SPE, Leica, Germany).
Analysis of lysosomal degradation capacity
DQ-BSA-Green was used to determine the lysosomal degradation capacity. Cells grown on coverslips in 24-well plates were incubated with 10 μg/ml of DQ-BSA-Green for 12 h (37 °C, 5% CO2), washed twice with PBS to remove excess probe and refreshed the medium. Then cells were treated with 0.5 μM Pb (12 h), EBSS medium (2 h) or 50 μM CQ (3 h), respectively. Slides were mounted and observed under a laser scanning confocal microscope with excitation set at 488 nm. Degradation capacity was measured by the green fluorescence signal released due to the degradation of DQ-BSA-Green.
Analysis of apoptosis by morphological changes and flow cytometry
Cells were pretreated with 2 μM CA 074 or 20 μM Pep A for 1 h, followed by 0.5 μM Pb treatment for another 12 h to assess its effect on Pb-induced apoptosis, respectively. Firstly, harvested cells under the indicated treatments were stained with annexin V/PI to analyze the distribution of apoptotic cells using flow cytometer. Secondly, DAPI staining was applied to assess the morphological changes of treated cells and 200 cells were randomly selected to count the apoptotic cells within every batch of experiment, each one performed in triplicate. Both of these two methods have been extensively described in our previous study.15
Data presentation
Experiments were performed at least three times with similar results. Data are presented as the mean±S.E.M. of the indicated number of replicates. Statistical comparisons were made using one-way analysis of variance (ANOVA) (Scheffe’s F test) after ascertaining the homogeneity of variance between the treatments, and P<0.05 was regarded as significant.
References
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ . Heavy metal toxicity and the environment. EXS 2012; 101: 133–164.
Ekong EB, Jaar BG, Weaver VM . Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int 2006; 70: 2074–2084.
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O . Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 2015; 30: 1235–1243.
Patrick L . Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev 2006; 11: 2–22.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016; 12: 1–222.
Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 2011; 22: 902–913.
Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z . Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 2012; 82: 1271–1283.
Sureshbabu A, Ryter SW, Choi ME . Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol 2015; 4: 208–214.
Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 2012; 8: 826–837.
Lv XH, Zhao DH, Cai SZ, Luo SY, You T, Xu BL et al. Autophagy plays a protective role in cell death of osteoblasts exposure to lead chloride. Toxicol Lett 2015; 239: 131–140.
Sui L, Zhang RH, Zhang P, Yun KL, Zhang HC, Liu L et al. Lead toxicity induces autophagy to protect against cell death through mTORC1 pathway in cardiofibroblasts. Biosci Rep 2015; 35: pii: e00186.
Song X, Li Z, Liu F, Wang Z, Wang L . Restoration of autophagy by puerarin in lead-exposed primary rat proximal tubular cells via regulating AMPK-mTOR signaling. J Biochem Mol Toxicol 2017; 31: e21869.
Villamil Giraldo AM, Appelqvist H, Ederth T, Öllinger K . Lysosomotropic agents: impact on lysosomal membrane permeabilization and cell death. Biochem Soc Trans 2014; 42: 1460–1464.
Liu WJ, Xu BH, Ye L, Liang D, Wu HL, Zheng YY et al. Urinary proteins induce lysosomal membrane permeabilization and lysosomal dysfunction in renal tubular epithelial cells. Am J Physiol Renal Physiol 2015; 308: F639–F649.
Wang L, Wang H, Hu M, Cao J, Chen D, Liu ZP . Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 2009; 83: 417–427.
Mizushima N, Yoshimori T, Levine B . Methods in mammalian autophagy research. Cell 2010; 140: 313–326.
Yang YP, Hu LF, Zheng HF, Mao CJ, Hu WD, **ong KP et al. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol Sin 2013; 34: 625–635.
Lu Y, Dong S, Hao B, Li C, Zhu K, Guo W et al. Vacuolin-1 potently and reversibly inhibits autophagosome-lysosome fusion by activating RAB5A. Autophagy 2014; 10: 1895–1905.
Ganley IG, Wong PM, Gammoh N, Jiang X . Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell 2011; 42: 731–743.
Boya P, Kroemer G . Lysosomal membrane permeabilization in cell death. Oncogene 2008; 27: 6434–6451.
Mindell JA . Lysosomal acidification mechanisms. Annu Rev Physiol 2012; 74: 69–86.
Vázquez CL, Colombo MI . Assays to assess autophagy induction and fusion of autophagic vacuoles with a degradative compartment, using monodansylcadaverine (MDC) and DQ-BSA. Methods Enzymol 2009; 452: 85–95.
Turk B, Turk D, Turk V . Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta 2000; 1477: 98–111.
Duann P, Lianos EA, Ma J, Lin PH . Autophagy, innate immunity and tissue repair in acute kidney injury. Int J Mol Sci 2016; 17: pii: E662.
Kaushal GP, Shah SV . Autophagy in acute kidney injury. Kidney Int 2016; 89: 779–791.
Liu F, Li ZF, Wang ZY, Wang L . Role of subcellular calcium redistribution in regulating apoptosis and autophagy in cadmium-exposed primary rat proximal tubular cells. J Inorg Biochem 2016; 164: 99–109.
Kimura A, Ishida Y, Nosaka M, Kuninaka Y, Hama M, Kawaguchi T et al. Exaggerated arsenic nephrotoxicity in female mice through estrogen-dependent impairments in the autophagic flux. Toxicology 2016; 339: 9–18.
Jiang P, Mizushima N . LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015; 75: 13–18.
Li M, Pi H, Yang Z, Reiter RJ, Xu S, Chen X et al. Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J Pineal Res 2016; 61: 353–369.
Kimura S, Noda T, Yoshimori T . Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007; 3: 452–460.
**e R, Nguyen S, McKeehan WL, Liu L . Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol 2010; 11: 89.
Hubert V, Peschel A, Langer B, Gröger M, Rees A, Kain R . LAMP-2 is required for incorporating syntaxin-17 into autophagosomes and for their fusion with lysosomes. Boil Open 2016; 5: 1516–1529.
Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J et al. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res 2013; 23: 508–523.
Luzio JP, Pryor PR, Bright NA . Lysosomes: fusion and function. Nat Rev Mol Cell Biol 2007; 8: 622–632.
Marshansky V, Rubinstein JL, Grüber G . Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 2014; 1837: 857–879.
Horng JL, Lin LY, Huang CJ, Katoh F, Kaneko T, Hwang PP . Knockdown of V-ATPase subunit A (atp6v1a) impairs acid secretion and ion balance in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 2007; 292: R2068–R2076.
van Hille B, Richener H, Schmid P, Puettner I, Green JR, Bilbe G . Heterogeneity of vacuolar H-ATPase: differential expression of two human subunit B isoforms. Biochem J 1994; 303: 191–198.
Ozaki N, Matheis KA, Gamber M, Feidl T, Nolte T, Kalkuhl A et al. Identification of genes involved in gentamicin-induced nephrotoxicity in rats-a toxicogenomic investigation. Exp Toxicol Pathol 2010; 62: 555–566.
Goyal S, Amar SK, Dubey D, Pal MK, Singh J, Verma A et al. Involvement of cathepsin B in mitochondrial apoptosis by p-phenylenediamine under ambient UV radiation. J Hazard Mater 2015; 300: 415–425.
Orr ME, Oddo S . Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther 2013; 5: 53.
Ivanova S, Repnik U, Bojic L, Petelin A, Turk V, Turk B . Lysosomes in apoptosis. Methods Enzymol 2008; 442: 183–199.
Aits S, Jäättelä M, Nylandsted J . Methods for the quantification of lysosomal membrane permeabilization: a hallmark of lysosomal cell death. Methods Cell Biol 2015; 126: 261–285.
Chwieralski CE, Welte T, Bühling F . Cathepsin-regulated apoptosis. Apoptosis 2006; 11: 143–149.
Hanzel CE, Almerira Gubiani MF, Verstraeten SV . Endosomes and lysosomes are involved in early steps of Tl(III)-mediated apoptosis in rat pheochromocytoma (PC12) cells. Arch Toxicol 2012; 86: 1667–1680.
Balboula AZ, Yamanaka K, Sakatani M, Kawahara M, Hegab AO, Zaabel SM et al. Cathepsin B activity has a crucial role in the developmental competence of bovine cumulusoocyte complexes exposed to heat shock during in vitro maturation. Reproduction 2013; 146: 407–417.
Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L . Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 2016; 90: 1193–1209.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 31472251), a foundation for the author of national excellent doctoral dissertation of PR China (No. 201266), the fund of Fok Ying Tung Education Foundation (No. 141022) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADD). This manuscript is partly supported by Funds of Shandong “Double Tops” Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by K Blomgren
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Song, XB., Liu, G., Liu, F. et al. Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis 8, e2863 (2017). https://doi.org/10.1038/cddis.2017.262
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2017.262
- Springer Nature Limited
This article is cited by
-
Erbin accelerates TFEB-mediated lysosome biogenesis and autophagy and alleviates sepsis-induced inflammatory responses and organ injuries
Journal of Translational Medicine (2023)
-
Recent insights into autophagy and metals/nanoparticles exposure
Toxicological Research (2023)
-
HIF-1α upregulation exerts the antagonistic effect against angiogenesis inhibition in manganese deficiency-induced tibial dyschondroplasia of broiler chicks
Veterinary Research Communications (2022)
-
Sidt2 is a key protein in the autophagy-lysosomal degradation pathway and is essential for the maintenance of kidney structure and filtration function
Cell Death & Disease (2021)
-
Protective Effects of Chrysin Against Oxidative Stress and Inflammation Induced by Lead Acetate in Rat Kidneys: a Biochemical and Histopathological Approach
Biological Trace Element Research (2021)