Highlights
-
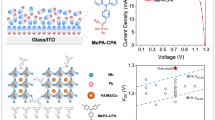
Three bulky cation chlorides (PMACl, PEACl and NMACl) are used to modify the perovskite surface and form pure-anion 2D (PMA)2PbCl4, mixed-anion 2D (PEA)2Pb(IxCl4-x), and non-2D NMAI passivation layers, respectively.
-
Intermolecular interactions between the bulky cations and the strength of cation-halide hydrogen bonds are critical to forming the three distinct passivation layers.
-
Semi-transparent wide-bandgap perovskite solar cells (WBG-PSCs) with ITO as the back electrode show hysteresis-free PCE of 18.60% and VOC deficit of 0.49 V.
Abstract
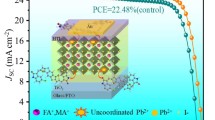
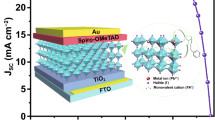
Wide-bandgap (WBG) perovskite solar cells suffer from severe non-radiative recombination and exhibit relatively large open-circuit voltage (VOC) deficits, limiting their photovoltaic performance. Here, we address these issues by in-situ forming a well-defined 2D perovskite (PMA)2PbCl4 (phenmethylammonium is referred to as PMA) passivation layer on top of the WBG active layer. The 2D layer with highly pure dimensionality and halide components is realized by intentionally tailoring the side-chain substituent at the aryl ring of the post-treatment reagent. First-principle calculation and single-crystal X-ray diffraction results reveal that weak intermolecular interactions between bulky PMA cations and relatively low cation-halide hydrogen bonding strength are crucial in forming the well-defined 2D phase. The (PMA)2PbCl4 forms improved type-I energy level alignment with the WBG perovskite, reducing the electron recombination at the perovskite/hole-transport-layer interface. Applying this strategy in fabricating semi-transparent WBG perovskite solar cells (indium tin oxide as the back electrode), the VOC deficits can be reduced to 0.49 V, comparable with the reported state-of-the-art WBG perovskite solar cells using metal electrodes. Consequently, we obtain hysteresis-free 18.60%-efficient WBG perovskite solar cells with a high VOC of 1.23 V.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Perovskite solar cells (PSCs) have made unprecedented development in the past few years with certified power conversion efficiency (PCE) soaring from 3.8% in 2009 to the current 25.7% [1,2,3,4,5,6]. Despite the rapid improvement, the PCE of single-junction PSCs is fundamentally limited by the Shockley-Queisser (S-Q) limit [7]. One of the most promising strategies for surpassing the S-Q limit is to construct perovskite-based tandem solar cells by stacking a wide-bandgap (WBG) perovskite top cell [8] on a narrow-bandgap bottom cell (e.g., crystalline silicon (c-Si) [42]. For the PVSK-NMACl film, XRD patterns (Fig. S5e-f) show a diffraction peak at 6.10°, which can be assigned to the non-2D NMAI salt phase, indicating ion exchange occurred during the post-treatment. Note that 2D perovskite is not formed because of the higher formation energy of NMA-based 2D perovskite [38].
The above GIWAXS and powder XRD results support that by tailoring the molecular structure of the bulky cation while kee** the halide unchanged (Cl–), the composition of the passivation layer on the WBG perovskite can be regulated. PMACl treatment transformed to pure-anion 2D passivation, PEACl treatment led to mixed-anion 2D passivation, and NMACl treatment led to non-2D passivation.
SEM images show that the surface morphology of perovskite treated with different organic ammonium salts changes significantly (Fig. S8). For the PVSK-PMACl sample, many regular small particles are observed on the surface and at the grain boundaries of WBG perovskite, which is most likely the formed 2D (PMA)2PbCl4. The surface of the PVSK-PEACl film is covered by large-size 2D (PEA)2PbIxCl4-x plates. However, the PVSK-NMACl film shows a much-disordered surface morphology. The surface roughness of different perovskite films was examined by atomic force microscopy (AFM). The root-mean-square (RMS) roughness of the control, PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl is 46.9, 37.9, 36.9, and 49.2 nm, respectively (Fig. S9). The decrease of roughness may be caused by the formation of 2D perovskites on the surface. The UV–vis absorption spectra of all perovskite films are almost the same (Fig. S10a), indicating that the passivating molecules do not change the perovskite lattice. The bandgap (Eg) of WBG perovskite determined by the Tauc plot is about 1.73 eV (Fig. S10b).
3.2 Characterizations of the Defect Passivation Effect
To study the passivation effect of different organic ammonium salts on the WBG perovskite, a series of characterizations of perovskite films and PSCs were carried out. Steady-state photoluminescence (PL) measurements (Fig. 2a) excited from the passivator side using the sample structure of glass/perovskite/passivator were performed to investigate the carrier recombination within the perovskite films. Compared with the control film, the PL intensity of PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films are significantly enhanced, indicating suppressed non-radiative recombination. Additionally, the emission peak of the PVSK-PMACl film shows a distinct blue shift, which can be attributed to the passivation of shallow trap states on the perovskite surface by the amino functional groups of PMACl [31]. To verify this effect, TRPL decay measurements were used to study the dynamic characteristics of photogenerated carriers. The results are shown in Fig. 2b, and the detailed parameters fitted by the bi-exponential function are summarized in Table S3. The PVSK-PMACl film exhibits the longest average lifetime (τavg) of 914.1 ns, while the τavg of control, PVSK-PEACl, and PVSK-NMACl films are 309.5, 675.9, and 369.4 ns, respectively. This result is consistent with the steady-state PL that PVSK-PMACl exhibits the highest PL intensity. In addition, PVSK-PMACl had the highest photoluminescence quantum efficiency (PLQY) value of 1.92% compared with other conditions (1.18% for Control, 1.69% for PVSK-PEACl, and 1.58% for PVSK-NMACl). These results imply that the pure-Cl 2D (PMA)2PbCl4 delivers the best defect passivation ability on the WBG perovskite. Space-charge-limited-current (SCLC) method was used to evaluate the trap density of perovskite films. Electron-only devices with the structure ITO/SnO2/Perovskite/[6, 6]-phenyl-C61-butyric acid methyl ester (PCBM)/Ag were prepared, and the dark J-V curves were measured (Fig. 2c). The trap-filled limit voltage (VTFL) is the voltage applied at the kink point, and the trap density can be calculated according to Eq. 1:
where Nt is the trap density, ε is the relative permittivity, ε0 is the vacuum permittivity, e is the elementary charge, and L is the thickness of the perovskite film. According to the equation, the VTFL is positively correlated with the trap density. As shown in Fig. 2c, the VTFL of the control film is 0.27 V, while the VTFL of PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl samples is reduced to 0.14, 0.16, and 0.19 V, respectively. Therefore, the corresponding trap density decreased from 1.6 × 1015 cm−3 of the control film to the minimum of 8.5 × 1014 cm−3 of the PVSK-PMACl. The trap density of PVSK-PEACl and PVSK-NMACl film is also slightly suppressed, which is 9.7 × 1014 and 1.2 × 1015 cm−3, respectively.
Characterization of defect passivation effect of PMACl, PEACl, and NMACl. a Steady-state photoluminescence spectra and b time-resolved photoluminescence decay curves of the control, PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films. c Space-charge-limited-current analysis of the electron-only devices measured under dark conditions. d Normalized transient photovoltage decay curves for the solar cells based on the control, PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films under light illumination with a power density of 100 mW cm–2. e Mott-Schottky analysis of PSCs in the dark using a frequency of 1 kHz. f Nyquist plots of the PSCs at a bias of 1.01 V under dark conditions. g EL-EQE of the devices while operating as LEDs. Insets: Photographs of the operating PSCs as LEDs. Schematic diagram of the energy-level alignment of WBG perovskite with h 2D (PMA)2PbCl4 and i 2D (PEA)2PbIxCl4-x
To explore the effect of the passivating molecules on the charge transport and carrier recombination within the PSCs, more characterizations were carried out on the device level. From the cross-sectional SEM of the device (Fig. S11), we find that perovskites treated with organic ammonium salts exhibit vertical columnar grain arrangement, which will facilitate carrier transport in the vertical direction. TPV decay curves were used to detect the carrier recombination inside the device. As shown in Fig. 2d, the carrier recombination lifetime (τr) is increased from 34.5 ms for the control to 192.9, 74.8, and 59.4 ms for the PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl film, respectively. These results indicate that the passivating layer mitigates the non-radiative recombination rate at the perovskite/hole-transport-layer interface. We also measured the built-in potentials (Vbi) of PSCs by Mott-Schottky analysis (Fig. 2e). Vbi of the devices based on the control, PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films are 0.90, 0.97, 0.92, and 0.92 V, respectively. Higher Vbi is conducive to more effective charge separation and reducing charge accumulation, which is beneficial to improving the photovoltage and reducing hysteresis. In addition, we further studied the carrier recombination behavior of the device by EIS (Fig. 2f). The PMACl passivated device shows the largest recombination resistance, indicating that the non-radiative recombination is effectively suppressed. The EQE of the PSCs working as light-emitting diodes (LEDs) can more directly reflect the non-radiative recombination, so we further measured the electroluminescence (EL) of the devices. The inserts of Fig. 2g are the photos of the lightened device. Due to the transparency of the back ITO electrode, both the top side and the bottom side can emit light. Figure 2g shows that the device based on PVSK-PMACl delivers the highest EL EQE, indicating the best effect of suppressing the non-radiative recombination. The corresponding EL spectra and J-V curves of the devices operating as LEDs are shown in Fig. S12. The above photophysics studies of the film and the carrier-transport studies of the devices support that the pure-Cl 2D (PMA)2PbCl4 possesses the best passivation effect on the WBG perovskite compared with the mixed I-Cl 2D (PEA)2PbIxCl4-x or the NMAI salt. In addition, XPS characterization was used to study the bonding environment on the surface of PVSK-PMACl film. From the C 1s spectra (Fig. S13a), it can be seen that the C=N characteristic peak of FA+ in PVSK-PMACl film shifts to lower binding energy, indicating that the benzene ring of PMACl provides an electron-rich environment for the 3D perovskite crystals. Moreover, in Fig. S13b, the characteristic peak of Pb 4f in the PVSK-PMACl film also shifts to lower binding energy than the control, indicating that Cl– of PMACl could interact with undercoordinated Pb2+. In other words, PMACl can fill halide/organic cation vacancies and coordinate with undercoordinated Pb2+ of the 3D perovskite to efficiently passivate the defects.
To study how the 2D structure affects the defect passivation ability of the WBG perovskite, ultraviolet photoelectron spectroscopy (UPS) was used to investigate the band alignment between the WBG 3D perovskite and different 2D perovskites (PMA)2PbCl4 and (PEA)2PbIxCl4-x. Combining the UPS spectra in Fig. S14 and the Tauc plots in Fig. S10b, the valence band maximum (VBM) and conduction band minimum (CBM) of 3D perovskites, (PMA)2PbCl4, (PEA)2PbIxCl4-x can be determined, and the energy level alignment is shown in Fig. 2h–i. The VBM of the WBG perovskite (− 5.59 eV) is higher than that of (PMA)2PbCl4 (− 6.05 eV) and (PEA)2PbIxCl4-x (− 5.86 eV), and the CBM of the WBG perovskite (− 3.86 eV) is lower than that of (PMA)2PbCl4 (− 2.50 eV) and (PEA)2PbIxCl4-x (− 3.54 eV), both form type-I band alignment with the WBG perovskite, which enables effective defect passivation ability [43]. (PMA)2PbCl4 has a larger bandgap of 3.55 eV than (PEA)2PbIxCl4-x (2.31 eV) and higher CBM, which can act as an electron-transport barrier and mitigate the electron–hole recombination at the perovskite/hole-transport-layer interface.
Based on the above analysis, PMACl, PEACl, and NMACl have defect passivation ability for PbI2-rich WBG perovskite films. Among them, the superior passivation ability of the PMACl treatment is most likely due to the united effects of the more ordered distribution of the 2D phase on the WBG perovskite surface and the improved interfacial energy-level arrangement. The performance of PVSK-PEACl is better than that of control and PVSK-NMACl, but inferior to that of PVSK-PMACl, the possible reason is that the formed 2D perovskite has passivation ability on the perovskite surface but also leads to undesirable aggregation. The performance of NMACl passivation is only better than that of control, mainly due to the passivation effect of the disordered NMAI salt formed on the perovskite surface being inferior. Therefore, the defect passivation ability is in the sequence of PVSK-PMACl > PVSK-PEACl > PVSK-NMACl > Control.
3.3 Molecular Mechanism of the Formation of Pure-Cl 2D Phase
To understand the molecular mechanism of how cation structure regulates the halide composition of the 2D passivating layer, first-principle calculations of the cations and single-crystal XRD measurements of the 2D perovskite were carried out. The calculated charge distribution reveals that the N atom in PEA+ possesses a more negative localized Mulliken charge (− 0.547) than PMA+ (− 0.531) (Fig. 3a-b), indicating larger electronegativity of the N atom in PEA+. This phenomenon is most likely due to the conjugation effect of the N atom in the benzyl group being stronger than that in the phenyl group. Therefore, PEA+ can more easily form hydrogen bonds with I– than that of PMA+, leading to a stable mixed I-Cl 2D phase. However, PMACl treatment results in a pure-Cl 2D phase due to unstable hydrogen bonding of N–H‧‧‧I. We prepared PMA-based and PEA-based 2D perovskites single crystals and measured their crystal structure. The crystal structure and the detailed crystal parameters are shown in Fig. 3c–f and Table 1. The crystal structure confirms that PEACl treatment forms a mixed I-Cl 2D phase while PMACl treatment forms a pure-Cl 2D phase. Symmetry reduction is observed from a Cmc21 space group of (PMA)2PbCl4 to a P-1 space group of (PEA)2PbIxCl4-x. Moreover, the side view of the crystal structure (Fig. 3c–f) shows that two adjacent PEA+ layers can form π–π interaction due to the paralleled stacking, which may further contribute to stabilizing the I-Cl mixed 2D phase. However, this π-π interaction may lead to undesirable aggregation of the passivator on the perovskite surface [44].
3.4 Performance of Semi-Transparent Wide-Bandgap Perovskite Solar Cells
The reduction of trap states and the favorable energy-level alignment are beneficial to suppressing the non-radiative recombination, thereby improving the photovoltaic performance of the WBG-PSCs. Figure 4a shows the J-V curves of the champion devices, and Table 2 summarizes the corresponding detailed photovoltaic parameters. Compared to the control with a PCE of 17.13% and a VOC of 1.18 V, the PCE of PSCs based on PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films are increased to 18.60%, 18.42%, and 18.12%, respectively. The corresponding VOC are increased to 1.23, 1.21, and 1.20 V, respectively. The performance of the device based on PVSK-PEACl is inferior to that of the device based on PVSK-PMACl. On the one hand, the large-size 2D perovskite formed by PEACl treatment is not well distributed in the grain boundaries; on the other hand, the mixed I-Cl 2D perovskite is insufficient in blocking the electrons, leading to partial carrier recombination. The device performance of PVSK-NMACl film is lower than that of PVSK-PMACl and PVSK-PEACl. One possible reason is probably that the disordered NMAI salt can’t deliver sufficient passivation. Incident photon-to-electron conversion efficiency (IPCE) spectra (Fig. S15) were measured, and the integrated short-circuit current density (JSC) of different devices was compared with the JSC extracted from the J-V curves (Table 2). The minor discrepancy confirmed the reliability of the J-V test. The statistical distribution of VOC, PCE, JSC and fill factor (FF) of 20 devices for each group show that the devices based on PVSK-PMACl possess the best performance, which reflects the superior passivation effect of the pure-Cl 2D phase (Figs. 4b-c and S16). The highest VOC of the PMACl-passivated device reaches 1.24 V, indicating a VOC deficit of 0.49 V for the 1.73-eV perovskite solar cells, which is one of the best performances among the semi-transparent WBG PSCs. The forward and reverse scanned J-V curves of the devices based on control, PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films are shown in Figs. 4d and S17. The hysteresis factor (HI) is calculated by Eq. 2:
and the detailed parameters are listed in Table S4. The HI of the control is 15.52% (PCEreverse of 16.52% and PCEforward of 14.00%), which is significantly reduced to 4.19% (PCEreverse of 18.38% and PCEforward of 17.62%) after PMACl treatment, indicating that the ion migration is probably suppressed after passivation. The performance of the semi-transparent device with the light incident from the MoOx/ITO side and the performance of the opaque device with a silver electrode is shown in Fig. S18 and Table S5. The performance difference between light incidents from the MoOx/ITO side and the glass/ITO side is due to different JSC. From Fig. S18b, the reduction of JSC on the MoOx/ITO side is mainly due to the absorption of short-wavelength light by the Spiro-OMeTAD. The semi-transparent cell with the light incident from the MoOx/ITO side and the opaque device obtained PCE of 16.42% and 19.62%, respectively. We note that in the 4-terminal (4T) tandem solar cells, the light can incident from the glass/ITO side of the semi-transparent cell, so the semi-transparent n-i-p device can be used to fabricate high-performance 4T tandems.
Photovoltaic performance of the WBG-PSCs. a Typical J-V curves of the WBG-PSCs based on the control, PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films under one-sun (100 mW cm–2) conditions. b, c Statistical results of VOC and PCE. Each group contains 20 PSCs. d J-V curves of the devices based on the control and PVSK-PMACl films measured by forward and reverse scans. e Steady-state power output at the maximum power point of the best device at 1.03 V for 300 s. f Stability of the WBG-PSCs. The devices were stored in the air with a relative humidity of ~ 10% and a temperature of ~ 25 °C
Stability is another parameter for evaluating the device performance. The short-term operational stability of devices is obtained at a fixed voltage near the maximum power point (MPP) under AM 1.5G solar simulator. As shown in Fig. 4e, the stabilized power output (SPO) of the optimal performance device is 18.10%, which is higher than that based on the control (16.33%), PVSK-PEACl (17.22%), and PVSK-NMACl (16.69%) (Fig. S19a–c), respectively. For long-term stability, we monitored the efficiency of the devices stored in the air (the relative humidity is ~ 10%, and the temperature is ~ 25 °C) for 50 days (Fig. 4f). The PCE of the control device drops to 73% of its initial PCE, while that of devices based on the PVSK-PMACl, PVSK-PEACl, and PVSK-NMACl films retain 94%, 90%, and 84% of their initial PCEs. To study the operational stability of the devices, the photovoltaic parameters of the devices operating at maximum power point (MPP) under 1 sun illumination were collected (Fig. S20). The results demonstrated that the operational stability of the devices was improved after passivation. The PVSK-PMACl device can maintain 80% of the initial efficiency after 350 h of MPP operation, while the control decays to 80% of the initial efficiency after 100 h. The much-improved stability of the PVSK-PMACl devices may originate from the reduced defect density and improved humidity resistance due to the formation of the pure-Cl 2D phase. In addition, the light transmission of the device was also tested. As shown in Fig. S21, the devices show good light transmission in the near-infrared region, which is comparable with the reported semi-transparent solar cells in high-performance perovskite/silicon tandem solar cells [45,46,47]. Therefore, our strategy is suitable for improving the performance of perovskite-based tandem solar cells.
4 Conclusions
In summary, we demonstrate that the halogen composition of the 2D perovskite passivation layer can be regulated by tailoring the structure of bulky ammonium cations. Due to the weak intermolecular interaction and relatively low cation-halide hydrogen bonding strength, the PMA+ forms a pure Cl-2D phase on the surface of WBG perovskite. In contrast, the PEA+ forms I-Cl mixed 2D perovskite, and the NMA+ forms non-2D perovskite (salt). The 2D (PMA)2PbCl4 perovskite has a large bandgap and a high conduction-band-minimum, which can form improved type-I energy level alignment with the 1.73-eV WBG perovskites. Therefore, the VOC deficits of semi-transparent WBG-PSCs (indium tin oxide as the back electrodes) can be reduced to 0.49 V, which is comparable with the reported state-of-the-art VOC deficits in WBG-PSCs with metal electrodes. Due to these effects, 18.60%-efficient semi-transparent WBG-PSCs are obtained. We believe this study will provide valuable insights into the designing principle of effective passivation strategies for WBG perovskites and perovskite-based tandem solar cells.
References
NREL Transforming energy (2023). https://www.nrel.gov/pv/cell-efficiency.html (Accessed Jan 1, 2023)
A. Kojima, K. Teshima, Y. Shirai, T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). https://doi.org/10.1021/ja809598r
Q. Jiang, Y. Zhao, X.W. Zhang, X.L. Yang, Y. Chen et al., Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 13, 460–466 (2019). https://doi.org/10.1038/s41566-019-0398-2
J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat et al., Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 592, 381–385 (2021). https://doi.org/10.1038/s41586-021-03406-5
J.J. Yoo, G. Seo, M.R. Chua, T.G. Park, Y. Lu et al., Efficient perovskite solar cells via improved carrier management. Nature 590, 587–593 (2021). https://doi.org/10.1038/s41586-021-03285-w
Y. Zhao, F. Ma, Z. Qu, S. Yu, T. Shen et al., Inactive (PbI2)2RbCl stabilizes perovskite films for efficient solar cells. Science 377, 531–534 (2022). https://doi.org/10.1126/science.abp8873
W. Shockley, H.J. Queisser, Detailed balance limit of efficiency of p-n junction solar cells. J. App. Phys. 32, 510–519 (1961). https://doi.org/10.1063/1.1736034
D.P. McMeekin, G. Sadoughi, W. Rehman, G.E. Eperon, M. Saliba et al., A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 351, 151–155 (2016). https://doi.org/10.1126/science.aad5845
Y. Hou, E. Aydin, M. De Bastiani, C. **ao, H. Isikgor Furkan et al., Efficient tandem solar cells with solution-processed perovskite on textured crystalline silicon. Science 367, 1135–1140 (2020). https://doi.org/10.1126/science.aaz3691
F.H. Isikgor, F. Furlan, J. Liu, E. Ugur, M.K. Eswaran et al., Concurrent cationic and anionic perovskite defect passivation enables 27.4% perovskite/silicon tandems with suppression of halide segregation. Joule 5, 1566–1586 (2021). https://doi.org/10.1016/j.joule.2021.05.013
L. Wang, Q. Song, F. Pei, Y. Chen, J. Dou et al., Strain modulation for light-stable n-i-p perovskite/silicon tandem solar cells. Adv. Mater. 34, 2201315 (2022). https://doi.org/10.1002/adma.202201315
A. Al-Ashouri, E. Köhnen, B. Li, A. Magomedov, H. Hempel et al., Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 370, 1300–1309 (2020). https://doi.org/10.1126/science.abd4016
T. Todorov, T. Gershon, O. Gunawan, Y.S. Lee, C. Sturdevant et al., Monolithic perovskite-CIGS tandem solar cells via in situ band gap engineering. Adv. Energy Mater. 5, 1500799 (2015). https://doi.org/10.1002/aenm.201500799
A. Al-Ashouri, A. Magomedov, M. Roß, M. Jošt, M. Talaikis et al., Conformal monolayer contacts with lossless interfaces for perovskite single junction and monolithic tandem solar cells. Energy Environ. Sci. 12, 3356–3369 (2019). https://doi.org/10.1039/C9EE02268F
R. Lin, J. Xu, M. Wei, Y. Wang, Z. Qin et al., All-perovskite tandem solar cells with improved grain surface passivation. Nature 603, 73–78 (2022). https://doi.org/10.1038/s41586-021-04372-8
J. Tong, Q. Jiang, A.J. Ferguson, A.F. Palmstrom, X. Wang et al., Carrier control in Sn–Pb perovskites via 2D cation engineering for all-perovskite tandem solar cells with improved efficiency and stability. Nat. Energy 7, 642–651 (2022). https://doi.org/10.1038/s41560-022-01046-1
L. Li, Y. Wang, X. Wang, R. Lin, X. Luo et al., Flexible all-perovskite tandem solar cells approaching 25% efficiency with molecule-bridged hole-selective contact. Nat. Energy 7, 708–717 (2022). https://doi.org/10.1038/s41560-022-01045-2
A new world record (2023). https://www.renshinesolar.com/page99?article_id=85 (Accessed Mar 7, 2023)
T. Leijtens, K.A. Bush, R. Prasanna, M.D. McGehee, Opportunities and challenges for tandem solar cells using metal halide perovskite semiconductors. Nat. Energy 3, 828–838 (2018). https://doi.org/10.1038/s41560-018-0190-4
F. Xu, M. Zhang, Z. Li, X. Yang, R. Zhu, Challenges and perspectives toward future wide-bandgap mixed-halide perovskite photovoltaics. Adv. Energy Mater. (2023). https://doi.org/10.1002/aenm.202203911
H. Tan, F. Che, M. Wei, Y. Zhao, M.I. Saidaminov et al., Dipolar cations confer defect tolerance in wide-bandgap metal halide perovskites. Nat. Commun. 9, 3100 (2018). https://doi.org/10.1038/s41467-018-05531-8
Y.-H. Lin, N. Sakai, P. Da, J. Wu, C. Sansom Harry et al., A piperidinium salt stabilizes efficient metal-halide perovskite solar cells. Science 369, 96–102 (2020). https://doi.org/10.1126/science.aba1628
M. Abdi-Jalebi, Z. Andaji-Garmaroudi, S. Cacovich, C. Stavrakas, B. Philippe et al., Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 555, 497–501 (2018). https://doi.org/10.1038/nature25989
Y. Lin, B. Chen, F. Zhao, X. Zheng, Y. Deng et al., Matching charge extraction contact for wide-bandgap perovskite solar cells. Adv. mater. 29, 1700607 (2017). https://doi.org/10.1002/adma.201700607
B. Chen, Z. Yu, K. Liu, X. Zheng, Y. Liu et al., Grain engineering for perovskite/silicon monolithic tandem solar cells with efficiency of 25.4%. Joule 3, 177–190 (2019). https://doi.org/10.1016/j.joule.2018.10.003
D. Kim, H.J. Jung, I.J. Park, B.W. Larson, S.P. Dunfield et al., Efficient, stable silicon tandem cells enabled by anion-engineered wide-bandgap perovskites. Science 368, 155–160 (2020). https://doi.org/10.1126/science.aba3433
C. Chen, Z. Song, C. **ao, R.A. Awni, C. Yao et al., Arylammonium-assisted reduction of the open-circuit voltage deficit in wide-bandgap perovskite solar cells: the role of suppressed ion migration. ACS Energy Lett. 5, 2560–2568 (2020). https://doi.org/10.1021/acsenergylett.0c01350
D.H. Kim, C.P. Muzzillo, J.H. Tong, A.F. Palmstrom, B.W. Larson et al., Bimolecular additives improve wide-band-gap perovskites for efficient tandem solar cells with cigs. Joule 3, 1734–1745 (2019). https://doi.org/10.1016/j.joule.2019.04.012
T. Bu, J. Li, Q. Lin, D.P. McMeekin, J. Sun et al., Structure engineering of hierarchical layered perovskite interface for efficient and stable wide bandgap photovoltaics. Nano Energy 75, 104917 (2020). https://doi.org/10.1016/j.nanoen.2020.104917
C. Chen, Z. Song, C. **ao, D. Zhao, N. Shrestha et al., Achieving a high open-circuit voltage in inverted wide-bandgap perovskite solar cells with a graded perovskite homojunction. Nano Energy 61, 141–147 (2019). https://doi.org/10.1016/j.nanoen.2019.04.069
Z. Li, J. Zhang, S. Wu, X. Deng, F. Li et al., Minimized surface deficiency on wide-bandgap perovskite for efficient indoor photovoltaics. Nano Energy 78, 105377 (2020). https://doi.org/10.1016/j.nanoen.2020.105377
Z. Wang, Q. Lin, F.P. Chmiel, N. Sakai, L.M. Herz et al., Efficient ambient-air-stable solar cells with 2d–3d heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 6, 17135 (2017). https://doi.org/10.1038/nenergy.2017.135
S. Gharibzadeh, B.A. Nejand, M. Jakoby, T. Abzieher, D. Hauschild et al., Record open-circuit voltage wide-bandgap perovskite solar cells utilizing 2d/3d perovskite heterostructure. Adv. Energy Mater. 9, 1803699 (2019). https://doi.org/10.1002/aenm.201803699
X. Zheng, B. Chen, J. Dai, Y. Fang, Y. Bai et al., Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2, 17102 (2017). https://doi.org/10.1038/nenergy.2017.102
Y. Zhou, F. Wang, Y. Cao, J.-P. Wang, H.-H. Fang et al., Benzylamine-treated wide-bandgap perovskite with high thermal-photostability and photovoltaic performance. Adv. Energy Mater. 7, 1701048 (2017). https://doi.org/10.1002/aenm.201701048
L. Wang, Q. Song, F. Pei, Y. Chen, J. Dou et al., Strain modulation for light-stable n–i–p perovskite/silicon tandem solar cells. Adv. Mater. 34, 2201315 (2022). https://doi.org/10.1002/adma.202201315
J.Y. Ye, J. Tong, J. Hu, C. **ao, H. Lu et al., Enhancing charge transport of 2d perovskite passivation agent for wide-bandgap perovskite solar cells beyond 21%. Solar RRL 4, 2000082 (2020). https://doi.org/10.1002/solr.202000082
L.S. Liang, H.T. Luo, J.J. Hu, H. Li, P. Gao, Efficient perovskite solar cells by reducing interface-mediated recombination: a bulky amine approach. Adv. Energy Mater. 10, 2000197 (2020). https://doi.org/10.1002/aenm.202000197
P. Wang, B. Chen, R. Li, S. Wang, Y. Li et al., 2d perovskite or organic material matter? Targeted growth for efficient perovskite solar cells with efficiency exceeding 24%. Nano Energy 94, 106914 (2022). https://doi.org/10.1016/j.nanoen.2021.106914
C. Jiang, J. Zhou, H. Li, L. Tan, M. Li et al., Double layer composite electrode strategy for efficient perovskite solar cells with excellent reverse-bias stability. Nano-Micro Lett. 15, 12 (2022). https://doi.org/10.1007/s40820-022-00985-4
B.D. Zhao, S. Bai, V. Kim, R. Lamboll, R. Shivanna et al., High-efficiency perovskite-polymer bulk heterostructure light-emitting diodes. Nat. Photonics 12, 783–789 (2018). https://doi.org/10.1038/s41566-018-0283-4
J. Tong, Q. Jiang, F. Zhang, S.B. Kang, D.H. Kim et al., Wide-bandgap metal halide perovskites for tandem solar cells. ACS Energy Lett. 6, 232–248 (2021). https://doi.org/10.1021/acsenergylett.0c02105
Q. Chen, H. Zhou, T.B. Song, S. Luo, Z. Hong et al., Controllable self-induced passivation of hybrid lead iodide perovskites toward high performance solar cells. Nano Lett. 14, 4158–4163 (2014). https://doi.org/10.1021/nl501838y
B. Yang, J. Suo, F. Di Giacomo, S. Olthof, D. Bogachuk et al., Interfacial passivation engineering of perovskite solar cells with fill factor over 82% and outstanding operational stability on n-i-p architecture. ACS Energy Lett. 6, 3916–3923 (2021). https://doi.org/10.1021/acsenergylett.1c01811
Y. Yao, P. Hang, B. Li, Z. Hu, C. Kan et al., Phase-stable wide-bandgap perovskites for four-terminal perovskite/silicon tandem solar cells with over 30% efficiency. Small 18, 2203319 (2022). https://doi.org/10.1002/smll.202203319
D. Yang, X. Zhang, Y. Hou, K. Wang, T. Ye et al., 28.3%-efficiency perovskite/silicon tandem solar cell by optimal transparent electrode for high efficient semitransparent top cell. Nano Energy 84, 105934 (2021). https://doi.org/10.1016/j.nanoen.2021.105934
B. Chen, S.W. Baek, Y. Hou, E. Aydin, M. De Bastiani et al., Enhanced optical path and electron diffusion length enable high-efficiency perovskite tandems. Nat. Commun. 11, 1257 (2020). https://doi.org/10.1038/s41467-020-15077-3
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22179042, U21A2078, and 51902110), the Natural Science Foundation of Fujian Province (2020J06021 and 2020J01064).
Funding
Open access funding provided by Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, L., **, Y., Fang, Z. et al. Efficient Semi-Transparent Wide-Bandgap Perovskite Solar Cells Enabled by Pure-Chloride 2D-Perovskite Passivation. Nano-Micro Lett. 15, 111 (2023). https://doi.org/10.1007/s40820-023-01090-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-023-01090-w