Abstract
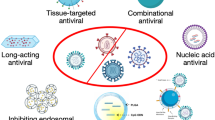
The host immune system is highly compromised in case of viral infections and relapses are very common. The capacity of the virus to destroy the host cell by liberating its own DNA or RNA and replicating inside the host cell poses challenges in the development of antiviral therapeutics. In recent years, many new technologies have been explored for diagnosis, prevention, and treatment of viral infections. Nanotechnology has emerged as one of the most promising technologies on account of its ability to deal with viral diseases in an effective manner, addressing the limitations of traditional antiviral medicines. It has not only helped us to overcome problems related to solubility and toxicity of drugs, but also imparted unique properties to drugs, which in turn has increased their potency and selectivity toward viral cells against the host cells. The initial part of the paper focuses on some important proteins of influenza, Ebola, HIV, herpes, Zika, dengue, and corona virus and those of the host cells important for their entry and replication into the host cells. This is followed by different types of nanomaterials which have served as delivery vehicles for the antiviral drugs. It includes various lipid-based, polymer-based, lipid–polymer hybrid–based, carbon-based, inorganic metal–based, surface-modified, and stimuli-sensitive nanomaterials and their application in antiviral therapeutics. The authors also highlight newer promising treatment approaches like nanotraps, nanorobots, nanobubbles, nanofibers, nanodiamonds, nanovaccines, and mathematical modeling for the future. The paper has been updated with the recent developments in nanotechnology-based approaches in view of the ongoing pandemic of COVID-19.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world has progressed in many realms, but viral diseases continue to exist and contribute to the mortality of humankind along with its varied socioeconomic manifestations. In recent times, there have been outbreaks of several viral infections caused by corona virus, Nipah virus, Ebola virus, Zika virus, dengue virus, chikungunya virus and different strains of influenza virus—H5N1 (avian flu) and H1N1 and H3N2 (swine flu). Recently, the novel coronavirus (nCoV) has caused severe pandemic claiming lives of approximately 2.1 lakh people so far, with high impact on socioeconomic implications around the world. In 2018, nineteen Nipah virus cases were reported in India, 17 of which resulted in mortality. Since 2001, the fatality rate due to Nipah virus infection has been reported to be between 68 and 100% in India [1]. Major outbreak of Ebola virus disease in West Africa during 2014–2016 claimed 11,315 lives out of 28,616 reported cases. In Australia, in 2019, in the first quarter itself, 27,540 notifications of influenza were received. Although decreased influenza activity is reported for the various continents across the world, different strains of the influenza virus are seen in the various parts of the world with the seasonal influenza A virus predominating [2]. Zika virus transmission has taken over an epidemic proportion in various parts of the world over the past few years. Presently, dengue is seen to afflict South East Asia 17 times more as compared to other viral infections, thus escalating the cost of dengue treatment to about $950 million [3]. Further, in May 2018, around 164,000 dengue incidences had struck globally [4]. Therefore, the economic ramifications associated with viral diseases have been quite high. Various risk factors identified for viral infections include environmental risks including water supply, sanitation facility and climate, life style including smoking and alcoholism, particular geographical area, various medical procedures like blood transfusion, surgery, transmission from vectors, etc. While from among these, a few factors are unavoidable and precautions need to be exercised to avoid them, efforts can be directed toward the others to elicit positive response [5,6,7].
The major challenge that remains in the development of effective antiviral agents is the ability of the virus to multiply in the host cell by liberating its own DNA or RNA. The host’s immune system is highly compromised in case of viral infections and relapses are very common. Also, due to the complexities associated with viruses, treatment is mostly symptomatic and complete eradication of the virus may not be possible. Distinguishing and diagnosing the exact type of viral disease is quite challenging. At times, due to past exposure, viral antibodies present in the host may get activated, rendering it difficult to detect incidental infections [8]. Difficulties faced in the prevention, detection, or treatment are seen as a red signal by the research community, and newer technologies have been explored to overcome the limitations of present therapies. The present review compiles recent advancements made in this direction and opens up new avenues for further research for diagnosis and treatment of viral infections.
General mechanisms of pathogenesis of viral infections
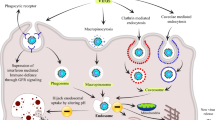
Most of the viral infections are subclinical, where the body’s defense mechanism arrests the course of infection before the clinical symptoms become apparent. Such infections are of great epidemiological significance as they become the means of spread of the virus through populations. Various stages of pathogenesis of viral disease include the following: (i) attachment of virus at point of entry, (ii) penetration into the host cell, (iii) uncoating of the virus, (iv) replication through transcription and translation leading to the synthesis of virus-specific proteins, (v) assembly of naked capsids through nucleocapsid, and (vi) release of virions resulting in further spread of infections [9]. Factors affecting pathogenesis mechanisms include accessibility of tissue to the causative virus, susceptibility of the cell to viral replication, and the resistance of the virus to the host defense mechanisms. The affinity of the virus to infect specific tissue depends on various factors like the presence of virus-specific receptors on the cell, cell transcription factors which enables the cell to recognize viral promoter and enhancer sequences, local pH, temperature, and presence of enzymes which may inactivate the virus [10]. Mechanisms adopted by the virus to cause destruction of the host cell involve blockade of cellular synthesis of macromolecules compromising cellular energy. Integration of the viral genome with the host genome causing mutations in the host genome is the indirect route to cell damage. The infection process is studied on the basis of virulence, virus-dependent factors, virulence genes, amount of inoculum, speed of replication, and degree to which the viral infection spreads [11]. The major issue linked with the study of viral diseases is that it is hard to evaluate the way in which the host defenses would interact with the virus. It may act by preventing the growth of the virus or it may stimulate immunological response in the affected tissue [12]. The basic structures of viruses, certain important proteins of the virus and the host cell which play a significant role in its entry and replication and spread, and currently available FDA-approved treatments and vaccines are presented in Table 1. A pictorial representation of these processes in influenza, dengue, Ebola, Zika virus, herpes infection, and HIV is made in Fig. 1.
Challenges of antiviral treatment
Continuous efforts in the direction of research on antiviral therapies have improved the quality of life of patients suffering from viral infections. However, the appearance of newer viral infections worldwide and the emergence of multidrug-resistant strains and its transmission have put forward greater challenges to the clinical utility of antiviral therapies.
-
(i)
Some antiviral drugs are known to interact with regular prescription medicines to give adverse drug–drug interactions [30]. Also, the toxic side effects are common outcomes of long-term treatment module, which may further hinder the patients from following their full medicinal regimen.
-
(ii)
Many of the antiviral drugs have short half-life, leading to an increase in the frequency of medication and poor patient compliance [31].
-
(iii)
Development of drug resistance is likely to occur, especially in immunocompromised patients as a result of prolonged drug exposure [32].
-
(iv)
Low bioavailability as a result of limited solubility or permeability may lead to administration of a higher dose and, consequently, may result in toxic effects [33].
-
(v)
Few viruses like HIV, Zika virus, and Ebola spread into inaccessible anatomical regions like the CNS, lymphatic system, and synovial fluid often making it difficult for the drugs to reach resulting in reduced therapeutic efficacy [34].
-
(vi)
Many viral infections remain in the latent stage for a prolonged period and their diagnosis and treatment poses challenges [35].
-
(vii)
Selectivity of antiviral agents toward the virus over the host cell and identification of the target which is unique to virus life cycle is another challenge in their development [36].
-
(viii)
Each virus is unique in its structure and function making the development of a broad-spectrum antiviral agent difficult [37].
Application of nanotechnology in antiviral therapeutics
With the advent of nanotechnology, it has been possible to comprehend the cellular mechanisms of the living cells and to develop relevant technologies which facilitate early diagnosis and treatment of various viral infections [38, 39]. Some of its applications consist of drug and gene delivery; use of fluorescent biological labels, detection of proteins, pathogens, and tumors; separation and purification of biological molecules and cells; tissue engineering; MRI contrast heightening; and pharmacokinetic studies [40,143].
Nanospheres
Nanospheres are smaller spherical structures of 10–200 nm diameter, where the drug is uniformly dispersed in the matrix system. This type of nanocarrier shows enhanced size-dependent characteristics, having the capability to prevent the drug from undergoing degradation. In addition, rapid drug clearance is observed due to smaller size. It offers site-specific delivery and the required drug release profiles. Recommended preparation techniques used are solvent evaporation, polymerization solvent displacement techniques, and phase inversion temperature methods [144]. Chitosan nanospheres loaded with acyclovir were synthesized using modified nanoemulsion template method for topical treatment of herpes. The spheres were of the average size of 200 nm and showed better permeation in in vitro skin permeation studies and higher potency against HSV-1 and HSV-2 than the free acyclovir itself [145].
Cyclodextrin-based delivery systems
Cyclodextrins (CDs) are cyclic oligosaccharides made of six to twelve α-d-glucopyranose monomers linked by α1–4 linkages having exceptional hydrophobic interior surfaces and hydrophilic rims with primary and secondary –OH groups. This type of molecular construct entraps the drug in a bucket-shaped cavity, thereby increasing the solubility of the drug and protecting the drug from degradation. Due to this, CDs become preferred delivery system for drugs [146]. The common native or parent cyclodextrins are α-CD, β-CD, and γ-CD comprising of 6, 7, and 8 glycopyranose units and molecular weights of 972, 1135, and 1297 Da, respectively [147]. These CDs have a homogeneous crystalline structure offering numerous advantages like their unique ability to interact with a range of organic and inorganic lipophilic molecules and form inclusion complexes. But their use is restricted due to low solubility of the CDs. Thus, alteration in CDs is being made to make them more suitable for their application in the pharmaceutical industry [148]. β-CD and its derivatives are more widely used than α-CD and γ-CD because of their safety and ease of production. The structural framework of β-CD is attractive with a height of 750–800 pm, external diameter of 1530 pm, internal diameter of 600–680 pm, and cavity volume of 260–265 Å3 [149]. These dimensions make the most ideal hosts for the formation of inclusion complexes. Chemical and enzymatic modifications of macrocycle l in CD derivatives that self-assemble in aqueous solutions provide different shapes of supramolecular nano-assemblies (vesicles, micelles, nanorods, nanospheres, and other kinds of nanoparticles and liquid crystalline structures of 30–500 nm in size depending on the concentration which are very useful for different types of nanodelivery systems) [150]. One of the common problems encountered with antiviral drugs is their poor bioavailability. A similar problem was found with the drug saquanavir. Couvreur and Vauthier formulated CDs loaded with saquanavir using poly(alkylcyanoacrylate) to deal with the issue. This improved solubility in water by 400-fold. Also, it was speculated that saquanavir could now bypass the efflux mechanism of P-gp, preventing its resistance [151]. Similarly, in another study, acyclovir was loaded with CDs using the copolymer such as Eudragit RLPO®, and on evaluation, it was found that intracellular uptake of the drug increased and it had sustained drug release over a period of 24 h [145]. Thus, by utilizing other drugs, CDs with different polymers can be formulated to increase the intracellular concentration of the drug.
Antimicrobial peptides with antiviral activities
Several peptides from natural (includes plants, arthropod venoms, amphibian skin, mammalian tissues) and microbial sources (includes bacteria, algae, and fungi) have been known to possess broad-spectrum antiviral activities. Various mechanisms, either targeting the virus or the host cells have been proposed for their antiviral activities. Some examples include magainin 1 and 2, dermaseptin S4 and temporin B (from frog skin), clavanin (from marine source), latarcin, protegrin (from swine WBCs), cyclotides (from plants), cecropin (from moth), defensins and cathelicidins (from mammals), and poly-γ-glutamic acid (from bacteria) exhibiting potent activity against various viruses like HIV, H1N1, DENV, and HCV [152, 153]. In addition, certain peptides, rationally designed and synthesized depending on the structure of the viral protein and its interaction with the host cell protein, have shown great potential as antiviral agents. Enfuvirtide is the first peptide antiviral drug approved against HIV [154]. Despite various advantages, various hurdles in their production, shorter half-life, and poor bioavailability have limited their use as antiviral agents. Nanotechnology-based solutions have been explored for the delivery of antiviral peptides. Peptide–nanoparticle conjugate systems have been extensively studied. Emileh et al. have reported gold nanoparticle–peptide triazole conjugates to be active against HIV-1 by disrupting the interactions between host receptor proteins and trimeric envelope spike glycoprotein of virus [155]. Recently, Alghrair et al. functionalized silver and gold nanoparticles with an antiviral peptide FluPep and reported increased antiviral potency against influenza A virus [156].
Table 3 compiles a list of polymer-based nanoformulations for antiviral treatment.
Carbon-based nanoformulation
Carbon-based nanoformulations are comprise of carbon nanotubes, graphene oxide nanoparticles, and fullerenes.
Carbon nanotubes
Carbon nanotubes (CNTs) are cylindrical-shaped hollow nanomaterials, viewed as tubes made by rolling up of planar graphene sheets. They can be viewed as coming from the rolling up of a graphene sheet, named as single-walled carbon nanotubes (SWCNTs), or a series of concentric rolled-up graphene sheets termed as multi-walled carbon nanotubes (MWCNTs) [157]. The cylindrical structure is capped with fullerene sheets on one end or both ends. The sp2-hybridized carbon atoms in graphene sheets impart a unique strength to CNTs. In addition, they display other unique characteristics like high aspect ratio, high surface area, cell penetration capacity, and ultralight weight [158]. The chemical vapor deposition (CVD) technique, laser-ablation technique, and electric arc-discharge techniques are commonly employed for the preparation of carbon nanotubes [159]. Though CNTs are widely explored for delivering chemotherapeutic agents at the target site, their overall application in the biomedical field is limited due to pulmonary toxicity and high hydrophobicity [160]. The proposed mechanisms for toxicity are uptaken by macrophages with subsequent generation of ROS and inflammatory mediators. However, functionalized CNTs have shown decreased toxicity and increased biodegradability. CNTs can be decorated with peptides, carbohydrates, and polymers and can be used for targeted therapy, when needed [161]. In one study, Kumar et al. stated about protoporphyrin IX (PPIX)-conjugated multi-walled nanotubes (MWNTs) and its ability to treat influenza using photodynamic therapy. It was found that in the presence of visible light, PPIX-MWNT may indulge in mechanisms like RNA strand breakage, protein oxidation, or protein–RNA cross-linking caused by reactive oxygen species (singlet oxygen and superoxide anion) leading to inactivation of the influenza viral strain. Probing into the inactivation mechanism of carbon nanotubes, they concluded that PPIX-MWNTs can be used for treating any viral infection as it displays nonspecificity in treating viral diseases. Also, PPIX-MWNT can be easily recovered through filtration and reused. Due to its multitarget mechanisms of antiviral action, it was proposed that PPIX-MWNTs have less chances of development of drug resistance [162].
Nanostructures have shown antiviral effect in respiratory syncytial virus, a virus causing severe bronchitis and asthma. The treatment is generally done by combining nanoparticles and gene-silencing technologies. In a novel approach, MWCNTs were functionalized with recombinant dengue virus 3 envelop proteins. This induced significant immune responses in mice [163]. Similarly, conjugation of functionalized CNTs with B and T cell peptide epitopes could generate a multivalent system that was able to induce a strong immune response; thus, CNTs were considered to be good candidates for vaccine delivery [164]. Further, functionalized CNTs were used for the transport of peptides (such as foot-and-mouth virus peptide) for vaccination [165].
Graphene
One of the most promising carbon-based nanomaterials with great potential for antiviral application is graphene. Graphene (G) is a two-dimensional (2-D) planar sheet of hexagonally arranged sp2-hybridized carbon atoms obtained from its three-dimensional (3-D) material of graphite [166]. It is chemically oxidized to graphene oxide (GO) to acquire oxygen bearing functional groups like hydroxyl, epoxide, and carboxylic acids [167]. Graphene-based nanomaterials (GBNs) have high surface area, high loading capacity, and superior mechanical strength which make them attractive candidates for carrying antiviral agents [168]. The oxygen-containing functional groups allow surface functionalization and conjugation strategies and show biocompatibility, reduced toxicity, and good dispersibility [169]. The amphiphilicity of GO makes the incorporation of hydrophilic as well as hydrophobic moieties possible [170]. In addition, these functional groups also provide attachment sites for various biological molecules like proteins, DNA, and RNA [171].
Recently, Pokhrel et al. studied the interactions between graphene and VP40 (viral matrix protein) of Ebola virus using molecular dynamics simulations and graphene pelleting assay. Graphene was found to interact strongly with VP-40 at various interfaces crucial for the formation of the viral matrix. They proposed the use of graphene-based nanoparticle solutions as disinfectant to prevent the Ebola epidemic [172].
In another study, 18 sulfonated magnetic nanoparticles were anchored onto reduced graphene oxide (SMRGO) sheets and used to trap and destroy HSV-1 photothermally, upon their irradiation with near-infrared light. It was found to be effective against 28 viral infections including HSV. It was found that SMRGO has higher entrapment efficiencies in comparison to magnetic nanoparticles due to increased entrap** efficiency, larger surface area, unique sheet-like structure, and outstanding photothermal characteristics shown by graphene [173].
Fullerenes
Fullerenes are among the first discoveries in symmetric carbon nanostructures and have received considerable attention in the case of antiviral research. Fullerenes are comprised fully of carbon atoms forming a nanosized caged hollow sphere. Buckminster fullerene (C60), also known as buckyball, is the most common form of fullerenes with 60 carbon atoms arranged in a spherical structure showing high symmetry [174]. Due to their unique architecture, immense scope of derivatization, free radical scavenging activity, and low toxicity, they are widely studied for drug delivery and antimicrobial and antiviral activities [175].
Fullerenes were found to fit inside the hydrophobic cavity of HIV proteases and inhibit HIV replication [176]. Structure–activity relationship studies revealed trans-position of substitutions and positive charge near the cage to be important for antiviral activity. Fulleropyrolidines with two ammonium groups have been shown to be active against HIV-1 and HIV-2 [177]. Also, fullerene C60 derivatized with two or more solubilizing side chains has been active when tested in CEM culture cells infected with HIV-1 and HIV-2 [178]. Additionally, amino-acid derivatives of fullerene C60 are found to inhibit HIV and HCV replication [179, 180].
Few studies aimed at screening fullerene derivatives for anti-influenza activity. Shoji et al. screened 12 fullerene derivatives for in vitro PA endonuclease inhibition. PA represents the subunit of influenza A RNA polymerase which demonstrates endonuclease activity. It was found that 8 fullerene derivatives demonstrated endonuclease inhibiting potential. In the MDCK cell culture system, these fullerene derivatives inhibited influenza A virus infection and expression of viral nucleoprotein [181].
Few of the studies also aimed at synthesizing anti-influenza fullerenes and evaluating their effectiveness against the influenza virus. Tollas and colleagues also prepared a fullerene conjugate having a thiosialosyl-a(2,6)-galactose disaccharide and evaluated to understand the multimeric interaction of a sialocluster with influenza virus NA and HA. Results revealed that these fullerene derivatives did not target HA but was able to target influenza NA slightly [182].
Table 4 gives an account of various carbon-based nanoformulations for antiviral treatment.
Inorganic-based nanoformulations
Quantum dots (QDs) (2–10 nm) are semiconductor nanocrystals having the shape of dots. They are comprised of a semiconductor core, overcoated by a shell, and a cap leading to improved solubility in aqueous buffers [190]. Fundamental semiconducting character and unique optical and electronic properties are attributed to the presence of the inorganic core consisting of semiconducting materials like silicon, cadmium selenide, cadmium sulfide, or indium arsenide [191]. Quantum dots find interesting applications in biomedical imaging due to its limited light scattering, narrow emission bands, and low tissue penetration. Quantum dots have been widely explored as theranostic platforms for simultaneous sensing, imaging, and therapy [192]. The advantages of quantum dots as a drug carrier system include improved bioavailability and stability of drugs, increased circulation times, active targeting, and localized therapy. In addition to this, QDs can be surface modified with targeting ligands [193, 194]. Yong et al. utilized saquinavir and transferrin (Tf)-conjugated quantum dots for the treatment of HIV. In vitro studies demonstrated that higher concentrations of saquinavir were able to cross the BBB by this method [195].
Metal and metal oxide nanoparticles have been widely explored for their antiviral activity. Among the various metal nanoparticles showing high efficacy are silver and gold, and among the various metal oxides are CuO, SiO2, TiO2, and CeO2. These nanoparticles have shown great efficacy against a broad spectrum of viruses like influenza (H3N2 and H1N1), HBV, HSV, HIV-1, HSV, dengue virus type-2, foot-and-mouth disease virus, and vesicular stomatitis virus [196].
Metal nanoparticles by virtue of their unique shape, size, structure, and local-field enhancement action can interact with viral surface proteins through Kazimir interaction and van der Waals forces causing its inactivation [197]. A variety of surface functionalizations with silane or thiol groups have shown to enhance interaction with biomolecules, affecting viral internalization in cells as well as the release of the drug molecule. Few researchers have also explored further grafting on functionalized metal nanoparticles in order to enhance their efficacy and selectivity [198].
Gold nanoparticles
Gold nanoparticles (AuNPs) are colloids of nanosized particles of gold. AuNPs show special optical properties in the presence of light. When AuNP comes in contact with light, the oscillating electromagnetic field of light triggers coherent oscillation of the free gold electrons [199]. This electron oscillation about the particle surface is responsible for a charge separation with regard to the ionic lattice, leading to a dipole oscillation in the path of the electric field of light. However, when the amplitude of the oscillation becomes maximum at a particular frequency, photons get confined to a small particle size and leads to a special phenomenon known as surface plasmon resonance (SPR) [200]. This SPR boosts all the radiative and nonradiative characteristics of the nanoparticles and, thus, has extensive application in areas of biological imaging, electronics, and materials science [200].
In 2015, Bayo et al. demonstrated that AuNP could enter the cells of various types like lymphocytes, macrophages, and brain microendothelial cells where HIV is known to replicate. Further, they modified raltegravir (RAL) by introducing a thiol group that served as a linker between RAL and AuNP. These particles inhibited HIV replication after penetrating inside infected primary PBMCs (peripheral blood mononuclear cells) exhausted from CD8+. Surprisingly, when the concentration of RAL was increased with the expectation of displaying high antiviral activity, it was found that anti-HIV activity was impaired. The experiment showed positive results when a low concentration of RAL was loaded into AuNP. The authors also performed an experiment using free AuNP and the results proved that it does not have any antiviral activity. So it was just a low concentration of RAL-loaded AuNP which turns it into an active compound having inhibitory action against HIV [201].
Small interfering RNAs (siRNAs) which can target particular viral gene can be employed in the treatment of dengue. However, siRNAs are prone to degradation by serum nucleases and to rapid elimination on account of its small size and anionic character. Paul et al. conjugated siRNAs with AuNPs and found that the complex had enhanced stability and could reduce dengue virus replication and the release of infectious virion in both pre- and post-infection conditions [202].
A breakthrough in the arena of gold nanoparticles was the design and synthesis of long and flexible linkers which mimicked heparan sulfate proteoglycans (HSPG), a target for viral attachment ligand. This allowed effective attachment of the virus to HSPG, generating strong forces that eventually lead to viral deformation. The mechanism was proposed by researchers on the basis of molecular dynamics simulations, electron microscopy images, and virucidal assays. These nanoparticles were nontoxic and effective against a broad range of viruses like HSV, human papilloma virus, respiratory syncytial virus (RSV), dengue, and lentivirus. Additionally, they were found to be active ex vivo in human cervicovaginal histocultures infected by HSV-2 and in vivo in mice infected with RSV [203].
Recently, Halder et al. synthesized highly monodispersed gold nanoparticles, stabilized by gallic acid. Reduction of AuCl4 using gallic acid was carried out using ultrasound-induced sonication which produced spherical nanoparticles in the range of 7–8 nm. These nanoparticles selectively inhibited HSV with EC50 of 32.3 μM in HSV-1 and 38.6 μM in HSV-2 with better safety profile compared to acyclovir. Prevention of viral cell attachment and penetration was proposed as a mechanism of action of these nanoparticles [204].
Silver nanoparticles
Silver metal possesses intrinsic antimicrobial activity due to its ability to interact with respiratory chain and electron transport chain enzymes and bacterial DNA. It has been extensively studied since ancient times for fighting infections [205]. Silver nanoparticles, on account of its small size and enormous surface area, facilitate rapid dissolution and have shown promising activity against a wide spectrum of viruses [206]. They demonstrate less chances of development of resistance on account of multiplicity of targets they act upon [207]. Nanoparticles of silver possess their own unique properties with regard to chemical stability, catalytic activity, high conductivity, and localized surface plasma resonance enabling researchers to envisage the implication of using AgNPs in disease therapeutics [208].
Galdiero et al. were the first authors to describe the antiviral activity of silver nanoparticles against HIV-1. They studied AgNP with three different surface functionalities: foamy carbon–coated AgNPs, poly(N-vinyl-2-pyrrolidone) (PVP)–coated AgNPs synthesized using glycerol as a reducing agent, and BSA-conjugated AgNPs. They proposed that the interaction between these NPs and the HIV viral surface glycoprotein gp120 was size dependent, as only the NPs in the size range of 1–10 nm were able to bind to the virus. BSA- and PVP-coated nanoparticles demonstrated lower inhibitory activity than silver nanoparticles released from the carbon matrix in in vitro assays on laboratory-adapted HIV-1 strain. AgNPs were shown to block the gp120–CD4 interaction. Additionally, they also were proved to inhibit the post-entry stages of infection by complexing with S and O of thiols and phosphates on amino acids and nucleic acid or directly bind to RNA or DNA, thus reducing the rate of reverse transcription [208]. Baram Pinto et al. synthesized mercaptoethane sulfonate (MES)–functionalized Au and AgNPs against HSV with a strategy to mimic heparan sulfate, present on the cell surface so that they compete for binding of the virus onto the cell. They inhibited HSV-1 infections by blocking the attachment and entry of the virus into the cell [209].
AgNPs have been investigated for their activity against H1N1 influenza virus. ** DNA-based COVID-19 vaccine | Waterloo Stories | University of Waterloo. [cited 2020 Jun 5]. Available from: https://uwaterloo.ca/stories/news/university-waterloo-develo**-dna-based-covid-19-vaccine ." href="/article/10.1007/s13346-020-00818-0#ref-CR297" id="ref-link-section-d59765347e5103">297].
Chinese researchers recently announced their success in develo** a special nanomaterial (nanozyme) that can absorb and deactivate this deadly virus with the efficiency of 96.5 to 99.9% [298].
Novavax, a US-based biotechnology company, has developed a vaccine candidate using its proprietary recombinant protein nanoparticle technology platform to generate antigens derived from the coronavirus spike (S) protein. Novavax expects to utilize its proprietary Matrix-M™ adjuvant with its COVID-19 vaccine candidate to enhance immune responses [299].
Forest business, nanoSeptic, is develo** a mineral nanocrystal-based technique to create a coating material which gets activated by any visible light to cause a strong oxidation reaction to completely breakdown any organic material including the virus. Similarly, a nanotech surface company in Italy has used titanium dioxide and silver ion–based nanoformulations to spray over buildings and surfaces to sanitize them. They claim that with this, the surfaces remain self-sterilized for years [300].
The Iranian government is supporting a manufacturing plant producing 4 million N95 masks per day based on nanofiber technology. They propose that nanofibers produced by electrospinning as perfect filter materials for manufacturing N95 masks. The Czech nanofiber technology firm Respilon Group has incorporated copper oxide into nanofibers to be used for producing mask that has the capability of trap** and destroying the virus. The Advanced Institute of Science and Technology (KAIST), Korea, developed nanofiber-based nanofilters using an insulation block electrospinning process which maintains its filtering efficiency even after 20 washes with ethanol. The orthogonal and unidirectional alignment of nanofibers is claimed to minimize the air pressure toward the filter and maximize filtration efficiency [301].
The World Nano Foundation (WNF) has created a second-generation rapid COVID-19 IgM/IgG antibody assay kit using gold nanoparticles into a testing strip. Also, an MIT spin out startup company has developed strips based on gold nanoparticles which could give the color reaction within 20 min of the start of the test. The strip is coated with antibodies that bind to the specific SARS-CoV2 viral protein and the second antibody is attached to gold nanoparticles. The patient’s sample is placed on the strip, and if it has viral antigen which binds to both these antibodies, a colored spot appears on the strip. Sona Nanotech Inc., a Canada-based company, is develo** a nanorod-based lateral flow test to screen the patient for the nCoV virus, which is expected to produce results within 5–15 min [302].
St. Olavs Hospital in Trondheim used iron oxide nanoparticles coated with silica to extract RNA from a patient’s sample in an attempt to fight the COVID-19 outbreak [303].
PreLynx in their portals have used vapors of a nanopolymer-based sanitizer which gets sprayed on individuals entering through this portal along with scanning of body temperature [304].
Nanoherbal formulations
Herbal drugs and phytoconstituents are considered to be safe and efficacious in comparison with allopathic medicines. Globally, the use of natural medicines is increasing. Principles of nanoscience and technology have been applied to these naturally occurring drugs for the purpose of increasing their bioavailability and achieving targeted delivery [305]. Table 8 gives a compilation of nanotechnology based herbal formulation for antiviral therapy.
Overcoming barriers and economization
Despite huge efforts and funds directed toward the development of nanotechnology-based formulations for various indications for the past three decades, there are only about twenty such FDA-approved products available in the market. The majority of these formulations are for cancer and neurological disorders, with liposomes and polymeric nanoparticles being largely used for drug delivery. However, the recent trend in investigational drugs is moving toward micellar systems, nanocrystals, dendrimers, and multicomponent targeted delivery systems and the therapeutic areas addressed are bacterial, fungal, and viral infections [306]. The next decade will witness the rise of nanotechnology platforms for the treatment of microbial infections including viral.
Nanotoxicity as a major limitation
With the rapid advancement of nanoscience in the healthcare sector, its adverse effects and toxicities are parallelly assessed by many scientists. On the virtue of the same reasons that lead to its increased potency, i.e., small size, larger surface area, specific shapes, and surface charges, their interaction at nonspecific target sites also increases [307]. The major mechanism responsible for the toxicity of nanomaterials is considered to be enhanced generation of oxidative stress and inflammatory mediators in various tissues which damage the biological molecules of the cell, namely proteins, lipids, and DNA [ Rajbari M, Rajbari N, Faridpur F. Morbidity and mortality due to Nipah or Nipah-like virus encephalitis in WHO South-East Asia Region Country: India 2018;2018. Available from: http://www.searo.who.int/entity/emerging_diseases/links/morbidity-and-mortality-nipah-sear-2001-2018.pdf. Immunisation Coalition 2019 Influenza statistics - Immunisation Coalition [Internet]. [cited 2019 Apr 12]. Available from: https://www.immunisationcoalition.org.au/news-media/2019-influenza-statistics/ S C, Mishra AK, Bazroy J. Trend of morbidity and mortality of dengue in Tamil Nadu and Puducherry, South India. Int J Community Med Public Heal. 2017;5:322. Available from: http://www.ijcmph.com/index.php/ijcmph/article/view/2291. CDTR-Disease, communicable report, threats. 2018;20–6. Available from: https://ecdc.europa.eu/sites/portal/files/documents/Communicable-disease-threats-report-26-may-2018_0.pdf. Lopez-Diez E, Perez S, Carballo M, Inarrea A, de la Orden A, Castro M, et al. Lifestyle factors and oncogenic papillomavirus infection in a high-risk male population. PLoS One. 2017;12:e0184492. Fuller TL, Calvet G, Estevam CG, Angelo JR, Abiodun GJ, Halai UA, et al. Behavioral, climatic, and environmental risk factors for Zika and chikungunya virus infections in Rio de Janeiro, Brazil, 2015-16. PLoS One. 2017;12:1–15. Reker C, Islam KM. Risk factors associated with high prevalence rates of hepatitis C infection in Egypt. Int J Infect Dis. 2014;25:104–6. https://doi.org/10.1016/j.ijid.2014.02.003. Zhu J-D, Meng W, Wang X-J, Wang H-CR. Broad-spectrum antiviral agents. Front Microbiol. 2015;6. Available from: http://www.frontiersin.org/Virology/10.3389/fmicb.2015.00517/abstract. Chinchar VG. Replication of viruses. Encycl Virol [Internet]. Elsevier; 1999. p. 1471–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/B0122270304002454 Baron S, Fons M AT. Viral pathogenesis medical microbiology. 4th edn. S, Baron. Manjarrez-zavala ME, Rosete-olvera DP, Gutiérrez-gonzález LH, Ocadiz-delgado R, Cabello-gutiérrez C. Pathogenesis of viral respiratory infection 2013; p. 3–32. Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–26. Available from: http://www.nature.com/articles/nri2802. Ferhadian D, Contrant M, Printz-Schweigert A, Smyth RP, Paillart J-C, Marquet R. Structural and functional motifs in influenza virus RNAs. Front Microbiol. 2018;9. Available from: http://journal.frontiersin.org/article/10.3389/fmicb.2018.00559/full. Yan X, Wang Q, Zhang Z, **e Y, Zhang H, Razi M, et al. Involvement of non-structural proteins (NS) in influenza A infection and viral tropism. Biochem Biophys Res Commun. Elsevier Inc.; 2012;428:62–7. Available from: https://doi.org/10.1016/j.bbrc.2012.10.006 Cooper NJ. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ. 2003;326:1235–1235. Available from: http://www.bmj.com/cgi/doi/10.1136/bmj.326.7401.1235. UYEKI TM. Influenza diagnosis and treatment in children: a review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J. 2003;22:164–77. Available from: https://insights.ovid.com/crossref?an=00006454-200302000-00015. Dahlke C, Kasonta R, Lunemann S, Krähling V, Zinser ME, Biedenkopf N, et al. Dose-dependent T-cell dynamics and cytokine cascade following rVSV-ZEBOV immunization. EBioMedicine. 2017. Merk A, Subramaniam S. HIV-1 envelope glycoprotein structure. Curr Opin Struct Biol. 2013;23:268–76. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0959440X13000493. Laskey SB, Siliciano RF. A mechanistic theory to explain the efficacy of antiretroviral therapy. Nat Rev Microbiol. 2014;12:772–80. https://doi.org/10.1038/nrmicro3351. De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents. 2009;33:307–20. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0924857908004846. Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–10. Gu M, Rice CM. Structures of hepatitis C virus nonstructural proteins required for replicase assembly and function. Curr Opin Virol. Elsevier B.V.; 2013;3:129–36. Available from: https://doi.org/10.1016/j.coviro.2013.03.013 Sager G, Gabaglio S, Sztul E, Belov G. Role of host cell secretory machinery in zika virus life cycle. Viruses. 2018;10:559. Available from: http://www.mdpi.com/1999-4915/10/10/559 Qin C, Qi J, Gao GF. Structures of the zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe. Elsevier Inc.; 2016;1–9. https://doi.org/10.1016/j.chom.2016.04.013 Reid S, Rimmer K, Thakur K. Zika virus and neurologic disease. Neurol Clin. Elsevier Inc; 2018;36:767–87. Available from: https://doi.org/10.1016/j.ncl.2018.06.003. Guabiraba R, Ryffel B. Dengue virus infection: current concepts in immune mechanisms and lessons from murine models. Immunology. 2014;141:143–56. Available from: http://doi.wiley.com/10.1111/imm.12188. Srivastava V. Quinacrine and berberine as antiviral agents against dengue and zika fever: in silico approach. 2018;2:2016–9. Osuna-Ramos JF, Reyes-Ruiz JM, del Ángel RM. The role of host cholesterol during flavivirus infection. Front Cell Infect Microbiol. 2018;8:1–12. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. Available from: http://www.nature.com/articles/s41421-020-0153-3. Oshikoya KA, Ogunleye, Lawal, Senbanjo S, Oreagba. Clinically significant interactions between antiretroviral and co-prescribed drugs for HIV-infected children: profiling and comparison of two drug databases. Ther Clin Risk Manag. 2013;215. Available from: http://www.dovepress.com/clinically-significant-interactions-between-antiretroviral-and-co-pres-peer-reviewed-article-TCRM. Gerber JG. Using pharmacokinetics to optimize antiretroviral drug-drug interactions in the treatment of human immunodeficiency virus infection. Clin Infect Dis. 2000;30:S123–9. Available from: http://academic.oup.com/cid/article/30/Supplement_2/S123/371450/Using-Pharmacokinetics-to-Optimize-Antiretroviral. Strasfeld L, Chou S. Antiviral drug resistance: mechanisms and clinical implications. Infect Dis Clin N Am. 2010;24:413–37. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0891552010000024. Singh R, Lilliard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–23. Vyas SP, Subhedar R, Jain S. Development and characterization of emulsomes for sustained and targeted delivery of an antiviral agent to liver. 2006;321–326. Chaudhuri A. Diagnosis and treatment of viral encephalitis. Postgrad Med J. 2002;78:575–83. Available from: http://pmj.bmj.com/cgi/doi/10.1136/pmj.78.924.575. Bule M, Khan F, Niaz K. Antivirals: past, present and future. Recent Adv Anim Virol. Singapore: Springer Singapore; 2019. p. 425–46. Available from: http://springer.longhoe.net/10.1007/978-981-13-9073-9_22. Adalja A, Inglesby T. Broad-spectrum antiviral agents: a crucial pandemic tool. Expert Rev Anti-Infect Ther. 2019;17:467–70. Available from: https://www.tandfonline.com/doi/full/10.1080/14787210.2019.1635009. Mendes PM. Cellular nanotechnology: making biological interfaces smarter. Chem Soc Rev. 2013;42:9207. Available from: http://xlink.rsc.org/?DOI=c3cs60198f. Villanueva-Flores F, Castro-Lugo A, Ramírez OT, Palomares LA. Understanding cellular interactions with nanomaterials: towards a rational design of medical nanodevices. Nanotechnology. 2020;31:132002. Cojocaru F-D, Botezat D, Gardikiotis I, Uritu C-M, Dodi G, Trandafir L, et al. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12:171. Available from: https://www.mdpi.com/1999-4923/12/2/171. Joo K-I, Lei Y, Lee C-L, Lo J, **e J, Hamm-Alvarez SF, et al. Site-specific labeling of enveloped viruses with quantum dots for single virus tracking. ACS Nano. 2008;2:1553–62. Available from: https://pubs.acs.org/doi/10.1021/nn8002136. Saini R, Saini S, Sharma S. Nanotechnology: The future medicine. J Cutan Aesthet Surg. 2010;3:32–3. Blecher K, Nasir A, Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence. 2011;2:395–401. Available from: http://www.tandfonline.com/doi/abs/10.4161/viru.2.5.17035. Puri A, Loomis K, Smith B, Lee J-H, Yavlovich A, Heldman E, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20402623. Mukherjee S, Ray S, Thakur R. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349 Available from: http://www.ijpsonline.com/text.asp?2009/71/4/349/57282. Nabi B, Rehman S, Baboota S, Ali J. Insights on oral drug delivery of lipid nanocarriers: a win-win solution for augmenting bioavailability of antiretroviral drugs. AAPS PharmSciTech. 2019;20:60 Available from: http://springer.longhoe.net/10.1208/s12249-018-1284-9. Attama AA, Momoh MA, Builders PF. Lipid nanoparticulate drug delivery systems: a revolution in dosage form design and development. Recent Adv Nov Drug Carr Syst. InTech; 2012. Available from: http://www.intechopen.com/books/recent-advances-in-novel-drug-carrier-systems/lipid-nanoparticulate-drug-delivery-systems-a-revolution-in-dosage-form-design-and-development. Li T, Cipolla D, Rades T, Boyd BJ. Drug nanocrystallisation within liposomes. J Control Release. 2018;288:96–110 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168365918305182. He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2018; Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211383517307311. Huang Z, Li X, Zhang T, Song Y, She Z, Li J, et al. Progress involving new techniques for liposome preparation. Asian J Pharm Sci. 2014;9:176–82 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1818087614000336. Mishra V, Bansal K, Verma A, Yadav N, Thakur S, Sudhakar K, et al. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics. 2018;10:191 Available from: http://www.mdpi.com/1999-4923/10/4/191. Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. Elsevier B.V.; 2015;495:439–46. Available from: https://doi.org/10.1016/j.ijpharm.2015.09.014. Guo D, Zhou T, Araínga M, Palandri D, Gautam N, Bronich T, et al. Creation of a long-acting nanoformulated 29,39-dideoxy-39-thiacytidine. J Acquir Immune Defic Syndr. Springer US. 2017;74:e75–83. https://doi.org/10.1038/s41467-018-02885-x. Müller RH, Alexiev U, Sinambela P, Keck CM. Nanostructured lipid carriers (NLC): the second generation of solid lipid nanoparticles. Percutaneous penetration enhancers chemical methods in penetration enhancement.. Dragicevic N, Maibach HI, editors. Berlin: Springer; 2016. Available from: http://springer.longhoe.net/10.1007/978-3-662-47862-2 Kuo Y-C, Chung J-F. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surfaces B Biointerfaces. 2011;83:299–306 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0927776510006685. Kanwar R, Rathee J, Salunke DB, Mehta SK. Green nanotechnology-driven drug delivery assemblies. ACS Omega. 2019;4:8804–15 Available from: https://pubs.acs.org/doi/10.1021/acsomega.9b00304. Kumar Gupta P, Bhandari N, Shah NH, Khanchandani V, Keerthana R, Nagarajan V et al. An update on nanoemulsions using nanosized liquid in liquid colloidal systems. Nanoemulsions - Prop Fabr Appl. IntechOpen; 2019. Available from: https://www.intechopen.com/books/nanoemulsions-properties-fabrications-and-applications/an-update-on-nanoemulsions-using-nanosized-liquid-in-liquid-colloidal-systems. Hobson JJ, Edwards S, Slater RA, Martin P, Owen A, Rannard SP. Branched copolymer-stabilised nanoemulsions as new candidate oral drug delivery systems. RSC Adv. 2018;8:12984–91 Available from: http://xlink.rsc.org/?DOI=C8RA01944D. Sutradhar KB, Amin ML. Nanoemulsions: increasing possibilities in drug delivery. Eur J Nanomed. 2013;5 Available from: https://www.degruyter.com/view/j/ejnm.2013.5.issue-2/ejnm-2013-0001/ejnm-2013-0001.xml. Manyarara TE, Khoza S, Dube A, Maponga CC. Formulation and characterization of a paediatric nanoemulsion dosage form with modified oral drug delivery system for improved dissolution rate of nevirapine. MRS Adv. 2018;3:2203–19 Available from: https://www.cambridge.org/core/product/identifier/S2059852118003201/type/journal_article. Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347:93–101. Prabhakar K, Afzal SM, Surender G, Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm Sin B. 2013;3:345–53 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211383513000774. M US, Lobo FJR, Uppuluri KB. Self nano emulsifying drug delivery systems for oral delivery of hydrophobic drugs. Biomed Pharmacol J. 2013;6:355–62 Available from: http://www.biomedpharmajournal.org/absdoic.php?snoid=425. Selvam RP, Kulkarni PK. Design and evaluation of self nanoemulsifying systems for poorly water soluble HIV drug. J PharmaSciTech. 2014;4:23–8 Available from: http://www.pharmascitech.in/admin/php/uploads/84_pdf.pdf. O’Keefe EP. siRNAs and shRNAs: tools for protein knockdown by gene silencing. Mater Methods. 2013;3 Available from: http://www.labome.com/method/siRNAs-and-shRNAs-Tools-for-Protein-Knockdown-by-Gene-Silencing.html. Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25:1467–75 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1525001617301119. Thi EP, Lee ACH, Geisbert JB, Ursic-Bedoya R, Agans KN, Robbins M, et al. Rescue of non-human primates from advanced Sudan ebolavirus infection with lipid encapsulated siRNA. Nat Microbiol. 2016;1:16142 Available from: http://www.nature.com/articles/nmicrobiol2016142. Leung AKK, Tam YYC, Cullis PR. Lipid nanoparticles for short interfering RNA delivery. Adv Genet Elsevier; 2014. https://doi.org/10.1016/B978-0-12-800148-6.00004-3. Nayak D, Boxi A, Ashe S, Thathapudi NC, Nayak B. Stavudine loaded gelatin liposomes for HIV therapy: preparation, characterization and in vitro cytotoxic evaluation. Mater Sci Eng C. Elsevier B.V. 2017;73:406–16. https://doi.org/10.1016/j.msec.2016.12.073. Jain S, Mistry MA, Swarnakar NK. Enhanced dermal delivery of acyclovir using solid lipid nanoparticles. Drug Deliv Transl Res. 2011;1:395–406 Available from: http://springer.longhoe.net/10.1007/s13346-011-0036-0. Seyfoddin A, Al-Kassas R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev Ind Pharm. 2013;39:508–19 Available from: http://www.tandfonline.com/doi/full/10.3109/03639045.2012.665460. McDonald TO, Giardiello M, Martin P, Siccardi M, Liptrott NJ, Smith D, et al. Antiretroviral solid drug nanoparticles with enhanced oral bioavailability: production, characterization, and in vitro-in vivo correlation. Adv Healthc Mater. 2014;3:400–11 Available from: http://doi.wiley.com/10.1002/adhm.201300280. Ravi PR, Vats R. Comparative pharmacokinetic evaluation of lopinavir and lopinavir-loaded solid lipid nanoparticles in hepatic impaired rat model. J Pharm Pharmacol. 2017;69:823–33. Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332218313222. FENG M. Liver targeting and anti-HBV activity of reconstituted HDL–acyclovir palmitate complex. Eur J Pharm Biopharm. 2008;68:688–93 Available from: http://linkinghub.elsevier.com/retrieve/pii/S0939641107002524. Kaushik S. Polymeric and ceramic nanoparticles: possible role in biomedical applications. Handb Polym Ceram Nanotechnol. Cham: Springer International Publishing; 2020. p. 1–17. Available from: http://springer.longhoe.net/10.1007/978-3-030-10614-0_39-1. Chiappetta DA, Hocht C, Taira C, Sosnik A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability. Nanomedicine. 2010;5:11–23 Available from: https://www.futuremedicine.com/doi/10.2217/nnm.09.90. Sawdon AJ, Peng C-A. Polymeric micelles for acyclovir drug delivery. Colloids Surfaces B Biointerfaces. 2014;122:738–45 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0927776514004330. Li Q, Du Y-Z, Yuan H, Zhang X-G, Miao J, Cui F-D, et al. Synthesis of lamivudine stearate and antiviral activity of stearic acid-g-chitosan oligosaccharide polymeric micelles delivery system. Eur J Pharm Sci. 2010;41:498–507 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928098710003015. Varshosaz J, Taymouri S, Jafari E, Jahanian-Najafabadi A, Taheri A. Formulation and characterization of cellulose acetate butyrate nanoparticles loaded with nevirapine for HIV treatment. J Drug Deliv Sci Technol. 2018;48:9–20 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1773224718306178. Martins C, Araújo F, Gomes MJ, Fernandes C, Nunes R, Li W, et al. Using microfluidic platforms to develop CNS-targeted polymeric nanoparticles for HIV therapy. Eur J Pharm Biopharm. Elsevier; 2018;0–1. https://doi.org/10.1016/j.ejpb.2018.01.014 Ogunwuyi O, Kumari N, Smith KA, Bolshakov O, Adesina S, Gugssa A, et al. Antiretroviral drugs-loaded nanoparticles fabricated by dispersion polymerization with potential for HIV/AIDS treatment. Infect Dis Res Treat. 2016;9:IDRT.S38108 Available from: http://journals.sagepub.com/doi/10.4137/IDRT.S38108. Kuo Y-C, Chung C-Y. Transcytosis of CRM197-grafted polybutylcyanoacrylate nanoparticles for delivering zidovudine across human brain-microvascular endothelial cells. Colloids Surfaces B Biointerfaces. 2012;91:242–9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0927776511006527. Cheung R, Ng T, Wong J, Chan W. Chitosan: an update on potential biomedical and pharmaceutical applications. Mar Drugs. 2015;13:5156–86 Available from: http://www.mdpi.com/1660-3397/13/8/5156. Panagopoulos P, Tsiodras S, Antoniadou A, Katsarolis I, Papadopoulos A, Poulakou G, et al. Efficacy and safety of an anti-retroviral combination regimen including either efavirenz or lopinavir–ritonavir with a backbone of two nucleoside reverse transcriptase inhibitors. Clin Microbiol Infect. 2006;12:486–9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1198743X14620164. Dhoke DM, Basaiyye SS, Khedekar PB. Development and characterization of L-HSA conjugated PLGA nanoparticle for hepatocyte targeted delivery of antiviral drug. J Drug Deliv Sci Technol. 2018;47:77–94 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1773224718303496. Gong Y, Chowdhury P, Midde NM, Rahman MA, Yallapu MM, Kumar S. Novel elvitegravir nanoformulation approach to suppress the viral load in HIV-infected macrophages. Biochem Biophys Reports. Elsevier B.V. 2017;12:214–9. https://doi.org/10.1016/j.bbrep.2017.10.005. Alka S. Development and evaluation of cyclodextrin based nanosponges for bioavailability enhancement of poorly bioavailable drug. World Jornal of Pharmacy and Pharmaceutical Sciences. 2017;6:805–36. Hassan H, Adam SK, Othman F, Shamsuddin AF, Basir R. Antiviral nanodelivery systems: current trends in acyclovir administration. J Nanomater. 2016;2016:1–8 Available from: https://www.hindawi.com/journals/jnm/2016/4591634/. Patel A, Shelat P, Lalwani A. Development and optimization of solid self-nanoemulsifying drug delivery system (S-SNEDDS) using Scheffe’s design for improvement of oral bioavailability of nelfinavir mesylate. Drug Deliv Transl Res. 2014;4:171–86 Available from: http://springer.longhoe.net/10.1007/s13346-014-0191-1. Sathigari S, Chadha G, Lee Y-HP, Wright N, Parsons DL, Rangari VK, et al. Physicochemical characterization of efavirenz–cyclodextrin inclusion complexes. AAPS PharmSciTech. 2009;10:81–7 Available from: http://www.springerlink.com/index/10.1208/s12249-008-9180-3. Lai SK, Wang Y-Y, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–71 Available from: http://linkinghub.elsevier.com/retrieve/pii/S0169409X08002652. Sun B, Yeo Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr Opin Solid State Mater Sci. 2012;16:295–301 Available from: http://linkinghub.elsevier.com/retrieve/pii/S135902861200054X. Kaur G, Aggarwal G, Harikumar SL. Nanosponge: new colloidal drug delivery system for topical delivery. Indo Global J Pharm Sci. 2015;5:53–7. Tejashri G, Amrita B, Darshana J. Cyclodextrin based nanosponges for pharmaceutical use: a review. Acta Pharm. 2013;63:335–58. Pothen LA, Thomas S. Encapsulation of zidovudine in PF-68 coated alginate conjugate nanoparticles for anti-HIV drug delivery. Int J Biol Macromol. Elsevier B.V.; 2017. https://doi.org/10.1016/j.ijbiomac.2017.09.078 Ratemi E. pH-responsive polymers for drug delivery applications. Stimuli Responsive Polym. Nanocarriers Drug Deliv. Appl. Vol. 1 Types Triggers. Elsevier Ltd.; 2018. https://doi.org/10.1016/B978-0-08-101997-9.00005-9 Bhaskar VV, Middha A, Srivastava P, Rajagopal S. Liquid chromatography/tandem mass spectrometry method for quantitative estimation of solutol HS15 and its applications. J Pharm Anal. 2015;5:120–9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2095177914000835. Kumar S, Dilbaghi N, Saharan R, Bhanjana G. Nanotechnology as emerging tool for enhancing solubility of poorly water-soluble drugs. Bionanoscience. 2012;2:227–50. Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844–92 Available from: https://pubs.acs.org/doi/10.1021/acs.chemrev.8b00199. Miyata K, Christie RJ, Kataoka K. Polymeric micelles for nano-scale drug delivery. React Funct Polym. 2011;71:227–34 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1381514810001835. Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–31 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169409X00001241. Mandal A, Bisht R, Rupenthal ID, Mitra AK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168365917300172. Gill KK, Kaddoumi A, Nazzal S. PEG–lipid micelles as drug carriers: physiochemical attributes, formulation principles and biological implication. J Drug Target. 2015;23:222–31 Available from: http://www.tandfonline.com/doi/full/10.3109/1061186X.2014.997735. Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine. 2010;5:485–505 Available from: https://www.futuremedicine.com/doi/10.2217/nnm.10.10. Jhaveri AM, Torchilin VP. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5 Available from: http://journal.frontiersin.org/article/10.3389/fphar.2014.00077/abstract. Kumari A, Singla R, Guliani A, Yadav SK. Nanoencapsulation for drug delivery. EXCLI J. 2014;13:265–86. Ahn YS, Baik HJ, Lee BR, Lee ES, Oh KT, Lee DH, et al. Preparation of multifunctional polymeric micelles for antiviral treatment. Macromol Res. 2010;18:747–52 Available from: http://springer.longhoe.net/10.1007/s13233-010-0802-8. Nagavarma BVN, Yadav HKS, Ayaz A, Vasudha LS, Shivakumar HG. Different techniques for preparation of polymeric nanoparticles-a review. Asian J Pharm Clin Res 2012;5(3):16-23. Verma G, Rajagopalan MD, Valluru R, Sridhar KA. Nanoparticles: a novel approach to target tumors. Nano- Microscale Drug Deliv Syst. Elsevier; 2017. p. 113–29. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780323527279000078. Singh A, Garg G, Sharma PK. Nanospheres: a novel approach for targeted drug delivery system. Int J Pharm Sci Rev Res. 2010;5:84–8. Ferrari R, Sponchioni M, Morbidelli M, Moscatelli D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale. 2018;10:22701–19 Available from: http://xlink.rsc.org/?DOI=C8NR05933K. Qian C, Chen Y, Feng P, **ao X, Dong M, Yu J, et al. Conjugated polymer nanomaterials for theranostics. Acta Pharmacol Sin. 2017;38:764–81 Available from: http://www.nature.com/articles/aps201742. Edagwa BJ, Zhou T, McMillan JM, Liu X-M, Gendelman HE. Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies. Curr Med Chem. 2014;21:4186–98 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25174930. Gunaseelan S, Gunaseelan K, Deshmukh M, Zhang X, Sinko PJ. Surface modifications of nanocarriers for effective intracellular delivery of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62:518–31 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169409X09003627. Ekladious I, Colson YL, Grinstaff MW. Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov. 2019;18:273–94 Available from: http://www.nature.com/articles/s41573-018-0005-0. Parveen S, Arjmand F, Tabassum S. Clinical developments of antitumor polymer therapeutics. RSC Adv. 2019;9:24699–721. Larson N, Ghandehari H. Polymeric conjugates for drug delivery. Chem Mater. 2012;24:840–53 Available from: https://pubs.acs.org/doi/10.1021/cm2031569. Chen X, Chen X, Chen W, Ma X, Huang J, Chen R. Extended peginterferon alfa-2a (Pegasys) therapy in Chinese patients with HBeAg-negative chronic hepatitis B. J Med Virol. 2014;86:1705–13 Available from: http://doi.wiley.com/10.1002/jmv.24013. Li J, Yu F, Chen Y, Oupický D. Polymeric drugs: advances in the development of pharmacologically active polymers. J Control Release. 2015;219:369–82 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168365915301425. Kjellén L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–75 Available from: http://www.annualreviews.org/doi/10.1146/annurev.bi.60.070191.002303. Basu A, Kanda T, Beyene A, Saito K, Meyer K, Ray R. Sulfated homologues of heparin inhibit hepatitis C virus entry into mammalian cells. J Virol. 2007;81:3933–41 Available from: https://jvi.asm.org/content/81/8/3933. Smith AAA, Kryger MBL, Wohl BM, Ruiz-Sanchis P, Zuwala K, Tolstrup M, et al. Macromolecular (pro)drugs in antiviral research. Polym Chem. 2014;5:6407–25 Available from: http://xlink.rsc.org/?DOI=C4PY00624K. Andersen AHF, Riber CF, Zuwala K, Tolstrup M, Dagnæs-Hansen F, Denton PW, et al. Long-acting, potent delivery of combination antiretroviral therapy. ACS Macro Lett. 2018;7:587–91 Available from: https://pubs.acs.org/doi/10.1021/acsmacrolett.8b00179. Tomalia DA. The dendritic state. Mater Today. 2005;8:34–46 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1369702105007467. Tripathy S, Das MK. Dendrimers and their applications as novel drug delivery carriers. J Appl Pharm Sci. 2013;3:142–9 Available from: http://www.japsonline.com/abstract.php?article_id=1064. Šebestík J, Reiniš M, Ježek J. Synthesis of dendrimers: convergent and divergent approaches. Biomed Appl Pept Glyco-Glycopeptide Dendrimers, Analog Dendrimeric Struct. Vienna: Springer Vienna; 2012. p. 55–81. Available from: http://springer.longhoe.net/10.1007/978-3-7091-1206-9_6. Abedi-Gaballu F, Dehghan G, Ghaffari M, Yekta R, Abbaspour-Ravasjani S, Baradaran B, et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–90 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2352940718301641. Garg T, Singh O, Arora S, Murthy RSR. Dendrimer - a novel scaffold for drug delivery. Int J Pharm Sci Rev Res. 2011;7:211–20. Kensinger RD, Catalone BJ, Krebs FC, Wigdahl B, Schengrund C-L. Novel polysulfated galactose-derivatized dendrimers as binding antagonists of human immunodeficiency virus type 1 infection. Antimicrob Agents Chemother. 2004;48:1614–23 Available from: https://aac.asm.org/content/48/5/1614. Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J Intern Med. 2014;276:579–617 Available from: http://doi.wiley.com/10.1111/joim.12280. Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, et al. SPL7013 gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. Goepfert PA, editor. PLoS One. 2011;6:e24095 Available from: https://dx.plos.org/10.1371/journal.pone.0024095. Telwatte S, Moore K, Johnson A, Tyssen D, Sterjovski J, Aldunate M, et al. Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1. Antiviral Res. 2011;90:195–9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0166354211002543. Sepúlveda-Crespo D, Gómez R, De La Mata FJ, Jiménez JL, Muñoz-Fernández MÁ. Polyanionic carbosilane dendrimer-conjugated antiviral drugs as efficient microbicides: recent trends and developments in HIV treatment/therapy. Nanomed Nanotechnol Biol Med. 2015;11:1481–98 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1549963415000829. Sepúlveda-Crespo D, Jiménez JL, Gómez R, De La Mata FJ, Majano PL, Muñoz-Fernández MÁ, et al. Polyanionic carbosilane dendrimers prevent hepatitis C virus infection in cell culture. Nanomed Nanotechnol Biol Med. 2017;13:49–58 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1549963416301307. Landers JJ, Cao Z, Lee I, Piehler LT, Myc PP, Myc A, et al. Prevention of influenza pneumonitis by sialic acid–conjugated dendritic polymers. J Infect Dis [Internet. 2002;186:1222–30 Available from: https://academic.oup.com/jid/article-lookup/doi/10.1086/344316. García-Gallego S, Díaz L, Jiménez JL, Gómez R, de la Mata FJ, Muñoz-Fernández MÁ. HIV-1 antiviral behavior of anionic PPI metallo-dendrimers with EDA core. Eur J Med Chem. 2015;98:139–48 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0223523415300532. Lancelot A, Clavería-Gimeno R, Velázquez-Campoy A, Abian O, Serrano JL, Sierra T. Nanostructures based on ammonium-terminated amphiphilic Janus dendrimers as camptothecin carriers with antiviral activity. Eur Polym J. 2017;90:136–49 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0014305716314689. Caminade A-M, Turrin C-O, Majoral J-P. Biological properties of phosphorus dendrimers. New J Chem. 2010;34:1512 Available from: http://xlink.rsc.org/?DOI=c0nj00116c. Yandrapu SK, Kanujia P, Chalasani KB, Mangamoori L, Kolapalli RV, Chauhan A. Development and optimization of thiolated dendrimer as a viable mucoadhesive excipient for the controlled drug delivery: an acyclovir model formulation. Nanomed Nanotechnol Biol Med. 2013;9:514–22 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1549963412006028. Martínez-Gualda B, Sun L, Rivero-Buceta E, Flores A, Quesada E, Balzarini J, et al. Structure-activity relationship studies on a Trp dendrimer with dual activities against HIV and enterovirus A71. Modifications on the amino acid. Antivir Res. 2017;139:32–40 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0166354216306222. Mayer C. Nanocapsules as drug delivery systems. Int J Artif Organs. 2005;28:1163–71. Parboosing R, Maguire GEM, Govender P, Kruger HG, Albert I, Central L, et al. Nanotechnology and the treatment of HIV infection. Viruses. 2012;4(4):488–520. Singh A, Garg G, Sharma PK. Review article. Nanospheres: a novel approach for targeted drug delivery system. Int J Pharm Sci Rev Res. 2010;5:84–8. Donalisio M, Leone F, Civra A, Spagnolo R, Ozer O, Lembo D, et al. Acyclovir-loaded chitosan nanospheres from nano-emulsion templating for the topical treatment of herpesviruses infections. Pharmaceutics. 2018;10:46 Available from: http://www.mdpi.com/1999-4923/10/2/46. Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–35. Miranda JCD, Martins TEA, Veiga F, Ferraz HG. Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drugs. Braz J Pharm Sci. 2011;47:665–81. Shelley H, Babu RJ. Role of cyclodextrins in nanoparticle based drug delivery systems. J Pharm Sci. American Pharmacists Association; 2018. https://doi.org/10.1016/j.xphs.2018.03.021. Voncina B, Vivo V. Cyclodextrins in Textile Finishing. Eco-friendly text dye finish. InTech; 2013. Available from: http://www.intechopen.com/books/eco-friendly-textile-dyeing-and-finishing/cyclodextrins-in-textile-finishing. Zerkoune L, Angelova A, Lesieur S. Nano-assemblies of modified cyclodextrins and their complexes with guest molecules: incorporation in nanostructured membranes and amphiphile nanoarchitectonics design. Nanomaterials. 2014;4:741–65 Available from: http://www.mdpi.com/2079-4991/4/3/741. Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006. Vilas Boas LCP, Campos ML, Berlanda RLA, de Carvalho Neves N, Franco OL. Antiviral peptides as promising therapeutic drugs. Cell Mol Life Sci. 2019;76:3525–42 Available from: http://springer.longhoe.net/10.1007/s00018-019-03138-w. Lee W, Lee S-H, Ahn D-G, Cho H, Sung M-H, Han SH, et al. The antiviral activity of poly-γ-glutamic acid, a polypeptide secreted by Bacillus sp., through induction of CD14-dependent type I interferon responses. Biomaterials [Internet. 2013;34:9700–8 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961213010302. Ding X, Zhang X, Chong H, Zhu Y, Wei H, Wu X, et al. Enfuvirtide (T20)-based lipopeptide is a potent HIV-1 cell fusion Inhibitor: implications for viral entry and inhibition. Kirchhoff F, editor. J Virol. 2017;91. Available from: https://jvi.asm.org/lookup/doi/10.1128/JVI.00831-17. Emileh A, Tuzer F, Yeh H, Umashankara M, Moreira DRM, LaLonde JM, et al. A model of peptide triazole entry inhibitor binding to HIV-1 gp120 and the mechanism of bridging sheet disruption. Biochemistry. 2013;52:2245–61 Available from: https://pubs.acs.org/doi/10.1021/bi400166b. Alghrair ZK, Fernig DG, Ebrahimi B. Enhanced inhibition of influenza virus infection by peptide–noble-metal nanoparticle conjugates. Beilstein J Nanotechnol. 2019;10:1038–47 Available from: https://www.beilstein-journals.org/bjnano/articles/10/104. Rahman G, Najaf Z, Mehmood A, Bilal S, Mian SA, Ali G. An overview of the recent progress in the synthesis and applications of carbon nanotubes. C—J Carbon Res. 2019;5:3. Kaur J, Gill GS, Jeet K. Applications of carbon nanotubes in drug delivery. Charact Biol Nanomater Drug Deliv. Elsevier; 2019. p. 113–35. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128140314000052. Szabó A, Perri C, Csató A, Giordano G, Vuono D, Nagy JB. Synthesis methods of carbon nanotubes and related materials. Materials (Basel). 2010;3:3092–140 Available from: http://www.mdpi.com/1996-1944/3/5/3092. Shao W, Arghya P, Yiyong M, Rodes L, Prakash S. Carbon nanotubes for use in medicine: potentials and limitations. Synth Appl carbon Nanotub their compos: InTech; 2013. Available from: http://www.intechopen.com/books/syntheses-and-applications-of-carbon-nanotubes-and-their-composites/carbon-nanotubes-for-use-in-medicine-potentials-and-limitations. Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N Carbon nanotubes: properties , synthesis , purification, and medical applications. Nanoscale Res Lett. 2014;9(1):393. Kumar R, Dhanawat M, Kumar S, Singh B, Pandit J, Sinha V. Carbon nanotubes: a potential concept for drug delivery applications. Recent Pat Drug Deliv Formul. 2014;8:12–26 Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1872-2113&volume=8&issue=1&spage=12. Versiani AF, Astigarraga RG, Rocha ESO, Barboza APM, Kroon EG, Rachid MA, et al. Multi-walled carbon nanotubes functionalized with recombinant dengue virus 3 envelope proteins induce significant and specific immune responses in mice. J Nanobiotechnol. 2017;15:26 Available from: http://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-017-0259-4. Andrade LM, Cox L, Versiani AF, da Fonseca FG. A growing world of small things: a brief review on the nanostructured vaccines. Future Virol. 2017;12:767–79 Available from: https://www.futuremedicine.com/doi/10.2217/fvl-2017-0086. Pantarotto D, Partidos CD, Hoebeke J, Brown F, Kramer E, Briand J-P, et al. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem Biol. 2003;10:961–6 Available from: https://linkinghub.elsevier.com/retrieve/pii/S107455210300214X. Casimir D, Alghamdi H, Ahmed YI, Garcia-Sanchez R, Misra P. Raman spectroscopy of graphene, graphite and graphene nanoplatelets. 2D Mater. IntechOpen; 2019. Available from: https://www.intechopen.com/books/2d-materials/raman-spectroscopy-of-graphene-graphite-and-graphene-nanoplatelets. Aliyev E, Filiz V, Khan MM, Lee YJ, Abetz C, Abetz V. Structural characterization of graphene oxide: surface functional groups and fractionated oxidative debris. Nanomaterials. 2019;9:1180 Available from: https://www.mdpi.com/2079-4991/9/8/1180. Donskyi IS, Azab W, Cuellar-Camacho JL, Guday G, Lippitz A, Unger WES, et al. Functionalized nanographene sheets with high antiviral activity through synergistic electrostatic and hydrophobic interactions. Nanoscale. 2019;11:15804–9 Available from: http://xlink.rsc.org/?DOI=C9NR05273A. Lategan K, Alghadi H, Bayati M, de Cortalezzi M, Pool E. Effects of graphene oxide nanoparticles on the immune system biomarkers produced by RAW 264.7 and human whole blood cell cultures. Nanomaterials. 2018;8:125 Available from: http://www.mdpi.com/2079-4991/8/2/125. Neto AJP, Fileti EE. Elucidating the amphiphilic character of graphene oxide. Phys Chem Chem Phys. 2018;20:9507–15. Dideikin AT, Vul’ AY. Graphene oxide and derivatives: the place in graphene family. Front Phys. 2019;6 Available from: https://www.frontiersin.org/article/10.3389/fphy.2018.00149/full. Pokhrel R, Sompornpisut P, Chapagain P, Olson B, Gerstman B, Pandey RB. Domain rearrangement and denaturation in Ebola virus protein VP40. AIP Adv. 2018;8:125129 Available from: http://aip.scitation.org/doi/10.1063/1.5063474. Deokar AR, Nagvenkar AP, Kalt I, Shani L, Yeshurun Y, Gedanken A, et al. Graphene-based “hot plate” for the capture and destruction of the herpes simplex virus type 1. Bioconjug Chem. 2017;28:1115–22. Goodarzi S, Da Ros T, Conde J, Sefat F, Mozafari M. Fullerene: biomedical engineers get to revisit an old friend. Mater Today. 2017;20:460–80 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1369702116301808. Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, et al. Medicinal applications of fullerenes. Int J Nanomed. 2007;2:639–49 Available from: http://www.ncbi.nlm.nih.gov/pubmed/18203430. Bondavalli P. Carbon and its new allotropes: fullerene, carbon nanotubes, and graphene. Graphene Relat Nanomater. Elsevier; 2018. p. 1–40. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780323481014000011. Martinez ZS, Castro E, Seong C-S, Cerón MR, Echegoyen L, Llano M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob Agents Chemother. 2016;60:5731–41 Available from: https://aac.asm.org/content/60/10/5731. Bosi S, Da Ros T, Spalluto G, Balzarini J, Prato M. Synthesis and anti-HIV properties of new water-soluble bis-functionalized[60]fullerene derivatives. Bioorg Med Chem Lett. 2003;13:4437–40 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0960894X03009703. Mashino T, Shimotohno K, Ikegami N, Nishikawa D, Okuda K, Takahashi K, et al. Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg Med Chem Lett. 2005;15:1107–9 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0960894X04014908. Marchesan S, Da Ros T, Spalluto G, Balzarini J, Prato M. Anti-HIV properties of cationic fullerene derivatives. Bioorg Med Chem Lett. 2005;15:3615–8 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0960894X05006244. Shoji M, Takahashi E, Hatakeyama D, Iwai Y, Morita Y, Shirayama R, et al. Anti-influenza activity of C60 fullerene derivatives. Digard P, editor. PLoS One. 2013;8:e66337 Available from: https://dx.plos.org/10.1371/journal.pone.0066337. Tollas S, Bereczki I, Borbás A, Batta G, Vanderlinden E, Naesens L, et al. Synthesis of a cluster-forming sialylthio-d-galactose fullerene conjugate and evaluation of its interaction with influenza virus hemagglutinin and neuraminidase. Bioorganic Med Chem Lett. Elsevier Ltd. 2014;24:2420–3. https://doi.org/10.1016/j.bmcl.2014.04.032. Iannazzo D, Pistone A, Galvagno S, Ferro S, De Luca L, Maria A, et al. Synthesis and anti-HIV activity of carboxylated and drug-conjugated multi-walled carbon nanotubes. Carbon N Y. Elsevier Ltd. 2014;3977464:1–14. https://doi.org/10.1016/j.carbon.2014.11.007. Yang XX, Li CM, Li YF, Wang J, Huang CZ. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–92 Available from: http://xlink.rsc.org/?DOI=C7NR06520E. Ye S, Shao K, Li Z, Guo N, Zuo Y, Li Q, et al. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces. 2015;7:21571–9 Available from: http://pubs.acs.org/doi/10.1021/acsami.5b06876. Iannazzo D, Pistone A, Ferro S, Luca L De, Romeo R, Buemi MR, et al. Graphene quantum dots based systems as HIV Inhibitors. Bioconjug Chem. 2018;29(9):3084-3093. Illescas BM, Rojo J, Delgado R, Mart N. Multivalent glycosylated nanostructures to inhibit Ebola virus infection. J Am Chem Soc. 2017:6018–25. Voronov II, Martynenko VM, Chernyak AV, Balzarini J, Schols D, Troshin PA. Synthesis and antiviral activity of water-soluble polycarboxylic derivatives of [60]fullerene loaded with 3,4-dichlorophenyl units. Chem Biodivers. 2018;15:e1800293 Available from: http://doi.wiley.com/10.1002/cbdv.201800293. Barras A, Pagneux Q, Sane F, Wang Q, Boukherroub R, Hober D, et al. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl Mater Interfaces. 2016;8:9004–13. Malik P, Gulati N, Malik RK, Nagaich U. Carbon nanotubes, quantum dots and dendrimers as potential nanodevices for nanotechnology drug delivery systems. Int J Pharm Sci NanotechInt J Pharm Sci Nanotech. 2013;6:2113–24. Klimov VI, Hollingsworth JA, Crooker SA, Kim H. Los Alamos National Security LLC 2007. Multifunctional nanocrystals. 2007. p. U.S. Patent 7, 261, 940. Matea C, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, et al. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomedicine. 2017;12:5421–31 Available from: https://www.dovepress.com/quantum-dots-in-imaging-drug-delivery-and-sensor-applications-peer-reviewed-article-IJN. Reshma VG, Mohanan PV. Quantum dots: applications and safety consequences. J Lumin. 2019;205:287–98 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022231318313334. Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:102 Available from: http://nanoscalereslett.springeropen.com/articles/10.1186/1556-276X-8-102. Yong K, Wang Y, Roy I, Rui H, Swihart MT, Law W, et al. Preparation of quantum dot / drug nanoparticle formulations for traceable targeted delivery and therapy. Theranostics. 2012;2(7):681. Yadavalli T, Shukla D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomed Nanotechnol Biol Med. 2017;13:219–30 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1549963416301289. Demchenko HO, Rusinchuk NM. Evaluation of the efficiency of interparticle interactions in nanosystems. J Nanotechnol. 2019;2019:1–8 Available from: https://www.hindawi.com/journals/jnt/2019/4270454/. Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65:1866–79. https://doi.org/10.1016/j.addr.2013.09.019. Guo J, Rahme K, He Y, Li L-L, Holmes J, O’Driscoll C. Gold nanoparticles enlighten the future of cancer theranostics. Int J Nanomedicine. 2017;12:6131–52 Available from: https://www.dovepress.com/gold-nanoparticles-enlighten-the-future-of-cancer-theranostics-peer-reviewed-article-IJN. Huang X, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2090123210000056. Bayo J, MP D, Martinez E. Medicinal chemistry. Future Med Chem. 2015;7:450–61. Paul AM, Shi Y, Acharya D, Douglas JR, Cooley A, Anderson JF, et al. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol. 2014;95:1712–22 Available from: http://jgv.microbiologyresearch.org/content/journal/jgv/10.1099/vir.0.066084-0. Cagno V, Andreozzi P, D’Alicarnasso M, Silva PJ, Mueller M, Galloux M, et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater. 2018;17:195–203. Halder A, Das S, Ojha D, Chattopadhyay D, Mukherjee A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater Sci Eng C Mater Biol Appl. 2018;89:413–21 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29752114. Rudramurthy G, Swamy M, Sinniah U, Ghasemzadeh A. Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules. 2016;21:836 Available from: http://www.mdpi.com/1420-3049/21/7/836. Reidy B, Haase A, Luch A, Dawson K, Lynch I. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials (Basel). 2013;6:2295–350 Available from: http://www.mdpi.com/1996-1944/6/6/2295. Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:32 Available from: http://springer.longhoe.net/10.1186/2228-5326-2-32. Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16:8894–918 Available from: http://www.mdpi.com/1420-3049/16/10/8894. Baram-Pinto D, Shukla S, Perkas N, Gedanken A, Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug Chem. 2009;20:1497–502 Available from: https://pubs.acs.org/doi/10.1021/bc900215b. **ang D, Chen Q, Pang L, Zheng C. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J Virol Methods. 2011;178:137–42 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0166093411003788. Li Y, Lin Z, Zhao M, Xu T, Wang C, Hua L, et al. Silver nanoparticle based codelivery of oseltamivir to inhibit the activity of the H1N1 influenza virus through ROS-mediated signaling pathways. ACS Appl Mater Interfaces. 2016;8:24385–93 Available from: https://pubs.acs.org/doi/10.1021/acsami.6b06613. Wang M, Marepally SK, Vemula PK, Xu C. Inorganic nanoparticles for transdermal drug delivery and topical application. Nanosci Dermatol. Elsevier; 2016. p. 57–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128029268000057. Zaid K. Alghrair DGF and Ebrahimi B. Enhanced inhibition of influenza virus infection by peptide-noble metal nanoparticle conjugates. BioR**v. 2018;15:2017–9. Available from: https://www.uam.es/gruposinv/meva/publicacionesjesus/capitulos_espanyol_jesus/2005_motivacionparaelaprendizajePerspectivaalumnos.pdf, https://www.researchgate.net/profile/Juan_Aparicio7/publication/253571379_Los_estudios_sobre_el_cambio_conceptual_. Szunerits S, Barras A, Khanal M, Pagneux Q, Boukherroub R. Nanostructures for the inhibition of viral infections. Molecules. 2015;20:14051–81 Available from: http://www.mdpi.com/1420-3049/20/8/14051. Antoine TE, Hadigal SR, Yakoub AM, Mishra YK, Bhattacharya P, Haddad C, et al. Intravaginal zinc oxide tetrapod nanoparticles as novel immunoprotective agents against genital herpes. J Immunol. 2016;196:4566–75 Available from: http://www.jimmunol.org/lookup/doi/10.4049/jimmunol.1502373. AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–90 Available from: http://pubs.acs.org/doi/10.1021/nn800596w. Kawata K, Osawa M, Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 2009;43:6046–51 Available from: http://pubs.acs.org/doi/abs/10.1021/es900754q. Lin Z, Li Y, Guo M, **ao M, Wang C, Zhao M, et al. Inhibition of H1N1 influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways. RSC Adv. Royal Society of Chemistry; 2017;7:35290–6. Available from: http://xlink.rsc.org/?DOI=C7RA06477B. Li Y, Lin Z, Guo M, Zhao M, **a Y, Wang C, et al. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int J Nanomedicine. 2018;13:2005–16. Hang X, Peng H, Song H, Qi Z, Miao X, Xu W. Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J Virol Methods. 2015;222:150–7 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0166093415002268. Vijayakumar S, Ganesan S. Gold nanoparticles as an HIV entry inhibitor. Curr HIV Res. 2012;10:643–6 Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-162X&volume=10&issue=8&spage=643. Tiwari P, Vig K, Dennis V, Singh S. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1:31–63 Available from: http://www.mdpi.com/2079-4991/1/1/31. Borker S, Patole M, Moghe A, Pokharkar V. Engineering of pectin-reduced gold nanoparticles for targeted delivery of an antiviral drug to macrophages: in vitro and in vivo assessment. Gold Bull. 2017;50:235–46 Available from: http://springer.longhoe.net/10.1007/s13404-017-0213-0. Kesarkar R, Yeole M, Dalvi B, Sharon M, Chowdhary A. Gold nanosphere intermediate for drug conjugation. Int J Pharm Sci Rev. 2015;31:143–6. Ga’al H, Fouad H, Tian J, Hu Y, Abbas G, Mo J. Synthesis, characterization and efficacy of silver nanoparticles against Aedes albopictus larvae and pupae. Pestic Biochem Physiol. 2018;144:49–56 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0048357517301232. Mori Y, Ono T, Miyahira Y, Nguyen VQ, Matsui T, Ishihara M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett. 2013;8:93 Available from: http://nanoscalereslett.springeropen.com/articles/10.1186/1556-276X-8-93. Mukherjee A, Waters AK, Kalyan P, Achrol AS, Kesari S, Yenugonda VM. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomed. 2019;14:1937–52 Available from: https://www.dovepress.com/lipid-polymer-hybrid-nanoparticles-as-a-next-generation-drug-delivery%2D%2Dpeer-reviewed-article-IJN. Dave V, Tak K, Sohgaura A, Gupta A, Sadhu V, Reddy KR. Lipid-polymer hybrid nanoparticles: synthesis strategies and biomedical applications. J Microbiol Methods. 2019;160:130–42 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167701219300533. Date T, Nimbalkar V, Kamat J, Mittal A, Mahato RI, Chitkara D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics Tushar. J Control Release. Elsevier B.V; 2017; Available from: https://doi.org/10.1016/j.jconrel.2017.12.016. Article R, Kumari L, Al HA, Sakure K, Badwaik HR. Recent advancements in the lipid polymer hybrid nanoparticles for drug delivery: an overview. Acta Sci Pharm Sci. 2019;3:21–8. Joshy KS, Snigdha S, Thomas A, George A, Kalarikkal N, Pothen LA, et al. Nanotechnology in HIV / AIDS: lipids and lipid hybrid systems-novel delivery approaches on antiviral drugs. JSM Nanotechnol Nanomed. 2018;6. Joshy KS, Snigdha S, Kalarikkal N, Pothen LA, Thomas S. Gelatin modified lipid nanoparticles for anti retroviral drug delivery. Chem. Phys. Lipids. Elsevier Ireland Ltd; 2017. https://doi.org/10.1016/j.chemphyslip.2017.07.002. Joshy KS, George A, Jose J, Kalarikkal N, Pothen LA, Thomas S. Novel dendritic structure of alginate hybrid nanoparticles for effective anti-viral drug delivery. Int J Biol Macromol. Elsevier B.V. 2017;103:1265–75. https://doi.org/10.1016/j.ijbiomac.2017.05.094. Joshy KS, George A, Snigdha S, Joseph B, Kalarikkal N, Pothen LA, et al. Novel core-shell dextran hybrid nanosystem for anti-viral drug delivery. Mater Sci Eng C. Elsevier B.V; 2018;#pagerange#. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928493117336962. Joshy KS, Snigdha S, Anne G, Nandakumar K, Laly AP, Sabu T. Poly (vinyl pyrrolidone)-lipid based hybrid nanoparticles for anti viral drug delivery. Chem Phys Lipids. 2018;210:82–9. ** K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8:23–33 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211383517303672. Vijayan V, Mohapatra A, Uthaman S, Park IK. Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics. 2019;11:534 Available from: https://www.mdpi.com/1999-4923/11/10/534. Schwarz B, Uchida M, Douglas T. Biomedical and catalytic opportunities of virus-like particles in nanotechnology. Adv Virus Res. 2017;97:1–60 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0065352716300501. Abdoli A, Soleimanjahi H, Kheiri MT, Jamali A, Sohani H, Abdoli M, et al. Reconstruction of H3N2 influenza virus based virosome in-vitro. Iran J Microbiol. 2013;5:166–71 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23825736. Parodi A, Molinaro R, Sushnitha M, Evangelopoulos M, Martinez JO, Arrighetti N, et al. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. Elsevier Ltd. 2017;147:155–68. https://doi.org/10.1016/j.biomaterials.2017.09.020. Yang G, Chen S, Zhang J. Bioinspired and biomimetic nanotherapies for the treatment of infectious diseases. Front Pharmacol. 2019;10 Available from: https://www.frontiersin.org/article/10.3389/fphar.2019.00751/full. Kanekiyo M, Wei C-J, Yassine HM, McTamney PM, Boyington JC, Whittle JRR, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499:102–6 Available from: http://www.nature.com/articles/nature12202. Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. 2019;176:1420–1431.e17 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867419301096. Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457–501 Available from: http://xlink.rsc.org/?DOI=C5CS00798D. Clawson C, Ton L, Aryal S, Fu V, Esener S, Zhang L. Synthesis and characterization of lipid–polymer hybrid nanoparticles with pH-triggered poly(ethylene glycol) shedding. Langmuir. 2011;27:10556–61 Available from: https://pubs.acs.org/doi/10.1021/la202123e. Du J, Lane LA, Nie S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J Control Release. 2015;219:205–14 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168365915300936. Hallan SS, Kaur P, Kaur V, Mishra N, Vaidya B. Lipid polymer hybrid as emerging tool in nanocarriers for oral drug delivery. Artif Cells Nanomed Biotechnol. 2014:1–16. Mahlumba P, Choonara Y, Kumar P, du Toit L, Pillay V. Stimuli-responsive polymeric systems for controlled protein and peptide delivery: future implications for ocular delivery. Molecules. 2016;21:1002 Available from: http://www.mdpi.com/1420-3049/21/8/1002. Li N, Yu M, Deng L, Yang J, Dong A. Thermosensitive hydrogel of hydrophobically-modified methylcellulose for intravaginal drug delivery. J Mater Sci Mater Med. 2012;23:1913–9 Available from: http://springer.longhoe.net/10.1007/s10856-012-4664-9. Shao P, Wang B, Wang Y, Li J, Zhang Y. The application of thermosensitive nanocarriers in controlled drug delivery. J Nanomater. 2011;2011:1–12 Available from: http://www.hindawi.com/journals/jnm/2011/389640/. Tian W, Han S, Huang X, Han M, Cao J, Liang Y, et al. LDH hybrid thermosensitive hydrogel for intravaginal delivery of anti-HIV drugs. Artif Cells Nanomed Biotechnol. 2019;47:1234–40 Available from: https://www.tandfonline.com/doi/full/10.1080/21691401.2019.1596935. Dalpiaz A, Pavan B. Nose-to-brain delivery of antiviral drugs: a way to overcome their active efflux? Pharmaceutics. 2018;10:39 Available from: http://www.mdpi.com/1999-4923/10/2/39. de Dios AS, Díaz-García ME. Multifunctional nanoparticles: analytical prospects. Anal Chim Acta. 2010;666:1–22 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003267010003211. Sanvicens N, Marco MP. Multifunctional nanoparticles - properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26:425–33. Alkubaisi NA, Aref NMA. Dispersed gold nanoparticles potentially ruin gold barley yellow dwarf virus and eliminate virus infectivity hazards. Appl Nanosci. 2017;7:31–40 Available from: http://springer.longhoe.net/10.1007/s13204-016-0540-0. Liu D, Lian Y, Fang Q, Liu L, Zhang J, Li J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int J Biol Macromol. 2018;116:1026–36 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0141813018306032. Lee M-Y, Yang J-A, Jung HS, Beack S, Choi JE, Hur W, et al. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano. 2012;6:9522–31 Available from: https://pubs.acs.org/doi/10.1021/nn302538y. Liu Y, Tan J, Thomas A, Ou-Yang D, Muzykantov VR. The shape of things to come: importance of design in nanotechnology for drug delivery. Ther Deliv. 2012;3:181–94 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22834196. Auría-Soro C, Nesma T, Juanes-Velasco P, Landeira-Viñuela A, Fidalgo-Gomez H, Acebes-Fernandez V, et al. Interactions of nanoparticles and biosystems: microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials. 2019;9:1365 Available from: https://www.mdpi.com/2079-4991/9/10/1365. Samuelsson LB, Bovbjerg DH, Roecklein KA, Hall MH. Sleep and circadian disruption and incident breast cancer risk: an evidence-based and theoretical review. Neurosci Biobehav Rev. 2018;84:35–48. https://doi.org/10.1016/j.neubiorev.2017.10.011. Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019:1–26 Available from: https://www.hindawi.com/journals/jnm/2019/3702518/. Mendel, J., 1999. Dispersions and coatings. Nanostructure Sci Technol. 1999;35–47 Springer, Dordrecht. Law W, Reynolds JL, Yong K. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int J Nanomedicine. 2012;7:5301–14. **ao K, Li Y, Luo J, Lee JS, **ao W, Gonik AM, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–46 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0142961211000342. Sharma A, Madhunapantula SV, Robertson GP. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin Drug Metab Toxicol. 2012;8:47–69 Available from: http://www.tandfonline.com/doi/full/10.1517/17425255.2012.637916. Kaur CD, Nahar M, Jain NK. Lymphatic targeting of zidovudine using surface-engineered liposomes. J Drug Target. 2008;16:798–805. Al-Halifa S, Gauthier L, Arpin D, Bourgault S, Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front Immunol. 2019;10 Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.00022/full. Ther JCS, Agarwal M. Role of nanovaccine in immunotherapy. J Cell Sci Ther. 2015;s8:8–11 Available from: https://www.omicsonline.org/open-access/role-of-nanovaccine-in-immunotherapy-2157-7013-S8-003.php?aid=61096. Chu SY, Vostiar I, Karki S, Moore GL, Lazar GA, Pong E, et al. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol Immunol. 2008;45:3926–33 Available from: http://www.ncbi.nlm.nih.gov/pubmed/18691763. Wang C, Zhu W, Luo Y, Wang B-Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomed Nanotechnol Biol Med. 2018;14:1349–60 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1549963418300698. Marques Neto LM, Kipnis A, Junqueira-Kipnis AP. Role of metallic nanoparticles in vaccinology: implications for infectious disease vaccine development. Front Immunol. 2017;8 Available from: http://journal.frontiersin.org/article/10.3389/fimmu.2017.00239/full. Deng L, Mohan T, Chang TZ, Gonzalez GX, Wang Y, Kwon Y-M, et al. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun. 2018;9:359 Available from: http://www.nature.com/articles/s41467-017-02725-4. Liu Q, Zheng X, Zhang C, Shao X, Zhang X, Zhang Q, et al. Conjugating influenza a (H1N1) antigen to n-trimethylaminoethylmethacrylate chitosan nanoparticles improves the immunogenicity of the antigen after nasal administration. J Med Virol. 2015;87:1807–15 Available from: http://doi.wiley.com/10.1002/jmv.24253. Dabaghian M, Latifi AM, Tebianian M, NajmiNejad H, Ebrahimi SM. Nasal vaccination with r4M2e.HSP70c antigen encapsulated into N-trimethyl chitosan (TMC) nanoparticulate systems: preparation and immunogenicity in a mouse model. Vaccine. 2018;36:2886–95 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0264410X18302597. Francica JR, Lynn GM, Laga R, Joyce MG, Ruckwardt TJ, Morabito KM, et al. Thermoresponsive polymer nanoparticles co-deliver RSV F trimers with a TLR-7/8 adjuvant. Bioconjug Chem. 2016;27:2372–2385. Available from: https://pubs.acs.org/doi/10.1021/acs.bioconjchem.6b00370. Ulery BD, Phanse Y, Sinha A, Wannemuehler MJ, Narasimhan B, Bellaire BH. Polymer chemistry influences monocytic uptake of polyanhydride nanospheres. Pharm Res. 2009;26:683–90 Available from: http://springer.longhoe.net/10.1007/s11095-008-9760-7. Hervé P-L, Deloizy C, Descamps D, Rameix-Welti M-A, Fix J, McLellan JS, et al. RSV N-nanorings fused to palivizumab-targeted neutralizing epitope as a nanoparticle RSV vaccine. Nanomed Nanotechnol Biol Med. 2017;13:411–20 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1549963416301149. Zhang S, Huang S, Lu L, Song X, Li P, Wang F. Curdlan sulfate–O-linked quaternized chitosan nanoparticles: potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination. Int J Nanomedicine. 2018;13:2377–94 Available from: https://www.dovepress.com/curdlan-sulfate-o-linked-quaternized-chitosan-nanoparticles-potential%2D%2Dpeer-reviewed-article-IJN. Ulery BD, Petersen LK, Phanse Y, Kong CS, Broderick SR, Kumar D, et al. Rational design of pathogen-mimicking amphiphilic materials as nanoadjuvants. Sci Rep. 2011;1:198 Available from: http://www.nature.com/articles/srep00198. Bohlmann GM. Process economic considerations for production of ethanol from biomass feedstocks. Ind Biotechnol. 2006;2:14–20 Available from: http://www.liebertpub.com/doi/10.1089/ind.2006.2.14. López-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ. Self-assembling protein nanoparticles in the design of vaccines. Comput Struct Biotechnol J. 2016;14:58–68 Available from: https://linkinghub.elsevier.com/retrieve/pii/S200103701530009X. Vicente S, Diaz-Freitas B, Peleteiro M, Sanchez A, Pascual DW, Gonzalez-Fernandez A, et al. A polymer/oil based nanovaccine as a single-dose immunization approach. Boyaka PN, editor. PLoS One. 2013;8:e62500 Available from: https://dx.plos.org/10.1371/journal.pone.0062500. Dacoba TG, Omange RW, Li H, Crecente-Campo J, Luo M, Alonso MJ. Polysaccharide nanoparticles can efficiently modulate the immune response against an HIV peptide antigen. ACS Nano. 2019;13:4947–59 Available from: https://pubs.acs.org/doi/10.1021/acsnano.8b07662. Yi Y, Lagniton PNP, Ye S, Li E, Xu R-H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–66 Available from: http://www.ijbs.com/v16p1753.htm. Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12 Available from: https://www.cureus.com/articles/29589-severe-acute-respiratory-syndrome-coronavirus-2-sars-cov-2-an-update. Su D, Lou Z, Sun F, Zhai Y, Yang H, Zhang R, et al. Dodecamer structure of severe acute respiratory syndrome coronavirus nonstructural protein nsp10. J Virol. 2006;80:7902–8 Available from: https://jvi.asm.org/content/80/16/7902. Fang SG, Shen H, Wang J, Tay FPL, Liu DX. Proteolytic processing of polyproteins 1a and 1ab between non-structural proteins 10 and 11/12 of coronavirus infectious bronchitis virus is dispensable for viral replication in cultured cells. Virology. 2008;379:175–80 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0042682208004340. Hofmann H, Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12:466–72 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0966842X04001878. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015;1282:1–23 Available from: http://springer.longhoe.net/10.1007/978-1-4939-2438-7_1. Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn. 2020:1–10 Available from: https://www.tandfonline.com/doi/full/10.1080/07391102.2020.1758788. Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0924857920300674. An Overview of Nanotechnology Patents Focusing on Coronaviruses | STATNANO. [cited 2020 Jun 5]. Available from: https://statnano.com/news/67513/An-Overview-of-Nanotechnology-Patents-Focusing-on-Coronaviruses. Dormont F, Brusini R, Cailleau C, Reynaud F, Peramo A, Gendron A, et al. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation. Sci Adv. 2020:eaaz5466 Available from: https://advances.sciencemag.org/lookup/doi/10.1126/sciadv.aaz5466. Arcturus reports positive preclinical data for its COVID-19 vaccine candidate | Arcturus Therapeutics, Inc.. [cited 2020 Jun 5]. Available from: https://ir.arcturusrx.com/news-releases/news-release-details/arcturus-reports-positive-preclinical-data-its-covid-19-vaccine. Alnylam® Pharmaceuticals. [cited 2020 Jun 5]. Available from: https://www.alnylam.com/. Sohrab SS, El-Kafrawy SA, Mirza Z, Kamal MA, Azhar EI. Design and delivery of therapeutic siRNAs: application to MERS-coronavirus. Curr Pharm Des. 2018;24:62–77 Available from: http://www.eurekaselect.com/156958/article. University of Waterloo develo** DNA-based COVID-19 vaccine | Waterloo Stories | University of Waterloo. [cited 2020 Jun 5]. Available from: https://uwaterloo.ca/stories/news/university-waterloo-develo**-dna-based-covid-19-vaccine. China leverages nanotechnology to deactivate the novel coronavirus | STATNANO. [cited 2020 Jun 5]. Available from: https://statnano.com/news/67544/China-Leverages-Nanotechnology-to-Deactivate-the-Novel-Coronavirus. Novavax awarded funding from CEPI for COVID-19 vaccine development | Novavax Inc. - IR Site. [cited 2020 Jun 5]. Available from: https://ir.novavax.com/news-releases/news-release-details/novavax-awarded-funding-cepi-covid-19-vaccine-development. Forest business working to prevent spread of coronavirus, products sent internationally | WSET. [cited 2020 Jun 5]. Available from: https://wset.com/news/local/forest-business-working-to-prevent-spread-of-coronavirus-products-sent-internationally. Iran leverages nanotechnology to launch West Asia’s largest face mask production plant | STATNANO. [cited 2020 Jun 5]. Available from: https://statnano.com/news/67581/Iran-Leverages-Nanotechnology-to-Launch-West-Asia’s-Largest-Face-Mask-Production-Plant. COVID-19 report & analysis — World Nano Foundation. [cited 2020 Jun 5]. Available from: https://www.worldnanofoundation.com/covid-19-report-analysis. Producing iron oxide nanoparticles for 150,000 COVID-19 tests per week. [cited 2020 Jun 6]. Available from: https://www.nanowerk.com/nanotechnology-news2/newsid=54893.php. Nanotechnology in battle against coronavirus | STATNANO. [cited 2020 Jun 2]. Available from: https://statnano.com/nanotechnology-in-battle-against-coronavirus. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres M d P, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71 Available from: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-018-0392-8. Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;42:742–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29234213. Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res Lett. 2018;13:44 Available from: https://nanoscalereslett.springeropen.com/articles/10.1186/s11671-018-2457-x. Fu PP, **a Q, Hwang H-M, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1021949814000064. Ahadian S, Radisic M. Nanotoxicity. Nanobiomaterials Sci Dev Eval. Elsevier; 2017. p. 233–48. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780081009635000124. Sereemaspun A, Rojanathanes R, Wiwanitkit V. Effect of gold nanoparticle on renal cell: an implication for exposure risk. Ren Fail. 2008;30:323–5. Antony JJ, Sivalingam PCB. Toxicological effects of silver nanoparticles. Environ Toxicol Pharmacol. 2015;40:729–32. Yoisungnern T, Choi Y-J, Han JW, Kang M-H, Das JGS. Internalization of silver nanoparticles into mouse spermatozoa results in poor fertilization and compromised embryo development. Sci Rep. 2015;5:11170. Gaillet S. RJ-MF 2015. Silver nanoparticles: their potential toxic effects after oral exposure and underlying mechanisms-a review. Chem Toxicol. 2015;77:58–63. Karlsson HL, Cronholm P, Gustafsson JML. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–32. ** CY, Zhu BS, Wang XF, et al. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem Res Toxicol. 2008;21:1871e7. Wang C-C, Wang S, **a Q, et al. Phototoxicity of zinc oxide nanoparticles in HaCaT keratinocytes e generation of oxidative DNA damage during UVA and visible light irradiation. J Nanosci Nanotechnol. 2013;13:3880e8. Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178:2587–600 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002944011002744. Gutiérrez-Praena D, Pichardo S, Sánchez E, Grilo A, Cameán AM, Jos A. Influence of carboxylic acid functionalization on the cytotoxic effects induced by single wall carbon nanotubes on human endothelial cells (HUVEC). Toxicol in Vitro. 2011;25:1883–8 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0887233311001639. Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano. 2013;7:2352–68 Available from: https://pubs.acs.org/doi/10.1021/nn305567s. Seabra AB, Paula AJ, de Lima R, Alves OL, Durán N. Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol. 2014;27:159–68 Available from: https://pubs.acs.org/doi/10.1021/tx400385x. Voigt N, Henrich-Noack P, Kockentiedt S, Hintz W, Tomas J, Sabel BA. Toxicity of polymeric nanoparticles in vivo and in vitro. J Nanopart Res. 2014;16:2379 Available from: http://springer.longhoe.net/10.1007/s11051-014-2379-1. Mourdikoudis S, Pallares RM, Thanh NTK. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018;10:12871–934 Available from: http://xlink.rsc.org/?DOI=C8NR02278J. Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6 Available from: https://www.frontiersin.org/article/10.3389/fchem.2018.00360/full. Shafagati N, Patanarut A, Luchini A, Lundberg L, Bailey C, Petricoin E, et al. The use of nanotrap particles for biodefense and emerging infectious disease diagnostics. Pathog Dis. 2014;71:164–76 Available from: https://academic.oup.com/femspd/article-lookup/doi/10.1111/2049-632X.12136. Jaworski E, Saifuddin M, Sampey G, Shafagati N, Van Duyne R, Iordanskiy S, et al. The use of nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells. Jacobson S, editor. PLoS One. 2014;9:e96778 Available from: http://dx.plos.org/10.1371/journal.pone.0096778. Shafagati N, Lundberg L, Baer A, Patanarut A, Fite K, Lepene B, et al. The use of nanotrap particles in the enhanced detection of Rift Valley fever virus nucleoprotein. Ikegami T, editor. PLoS One. 2015;10:e0128215 Available from: https://dx.plos.org/10.1371/journal.pone.0128215. Shafagati N, Narayanan A, Baer A, Fite K, Pinkham C, Bailey C, et al. The use of nanotrap particles as a sample enrichment method to enhance the detection of Rift Valley fever virus. Peters CJ, editor. PLoS Negl Trop Dis. 2013;7:e2296 Available from: https://dx.plos.org/10.1371/journal.pntd.0002296. Shafagati N, Fite K, Patanarut A, Baer A, Pinkham C, An S, et al. Enhanced detection of respiratory pathogens with nanotrap particles. Virulence. 2016;7:756–69. Cheng H, Chen J, Cai Z, Du L, Hou J, Qiao X, et al. Development of GEM-PA-nanotrap for purification of foot-and-mouth disease virus. Vaccine. 2019;37:3205–13 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0264410X19305663. Akhrymuk I, Lin S-C, Sun M, Patnaik A, Lehman C, Altamura L, et al. Magnetic nanotrap particles preserve the stability of Venezuelan equine encephalitis virus in blood for laboratory detection. Front Vet Sci. 2020;6 Available from: https://www.frontiersin.org/article/10.3389/fvets.2019.00509/full. Lin S-C, Carey BD, Callahan V, Lee J-H, Bracci N, Patnaik A, et al. Use of nanotrap particles for the capture and enrichment of Zika, chikungunya and dengue viruses in urine. Roques P, editor. PLoS One. 2020;15:e0227058 Available from: https://dx.plos.org/10.1371/journal.pone.0227058. Mavroidis C, Ferreira A, editors. Nanorobotics: current approaches and techniques: Springer; 2013. Manjunath A, Kishore V. The promising future in medicine: nanorobots. Biomed Sci Eng. 2014;2:42–7. Nistor MT, Rusu AG. Nanorobots with applications in medicine. Polym Nanomater Nanotherapeutics. Elsevier; 2019. p. 123–49. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128139325000030. Ravi Bandakkanavar. Anti-HIV using nanorobots - Krazytech. [cited 2018 Aug 11]. Available from: https://krazytech.com/technical-papers/anti-hiv-using-nano-robots. Cavalli R, Soster M, Argenziano M. Nanobubbles: a promising efficienft tool for therapeutic delivery. Ther Deliv. 2016;7:117–38 Available from: http://www.future-science.com/doi/10.4155/tde.15.92. Song L, Wang G, Hou X, Kala S, Qiu Z, Wong KF, et al. Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer. Acta Biomater. 2020;108:313–25 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1742706120301756. Prabhakar A, Banerjee R. Nanobubble liposome complexes for diagnostic imaging and ultrasound-triggered drug delivery in cancers: a theranostic approach. ACS Omega. 2019;4:15567–80 Available from: https://pubs.acs.org/doi/10.1021/acsomega.9b01924. Thakkar S, Misra M. Electrospun polymeric nanofibers: new horizons in drug delivery. Eur J Pharm Sci. 2017;107:148–67 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0928098717304001. Yarin AL, Pourdeyhimi B, Ramakrishna S. Fundamentals and applications of micro and nanofibers. Cambridge: Cambridge University Press; 2014. Available from: http://ebooks.cambridge.org/ref/id/CBO9781107446830. Carson D, Jiang Y, Woodrow KA. Tunable release of multiclass anti-HIV drugs that are water-soluble and loaded at high drug content in polyester blended electrospun fibers. Pharm Res. 2016;33:125–36 Available from: http://springer.longhoe.net/10.1007/s11095-015-1769-0. Novel nanofiber-based technology could help prevent HIV/AIDS transmission. [cited 2014 Nov 4]. Available from: https://www.sciencedaily.com/releases/2014/11/141104183713.htm. Wu Y, Weil T. Nanodiamonds for biological applications. Phys Sci Rev. 2017;2. Available from: http://www.degruyter.com/view/j/psr.2017.2.issue-6/psr-2016-0104/psr-2016-0104.xml. Moore LK, Gatica M, Chow EK, Ho D. Diamond-based nanomedicine: enhanced drug delivery and imaging. Disruptive Sci Technol. 2012;1:54–61 Available from: http://online.liebertpub.com/doi/abs/10.1089/dst.2012.0007. Roy U, Drozd V, Durygin A, Rodriguez J, Barber P, Atluri V, et al. Characterization of nanodiamond-based anti-HIV drug delivery to the brain. Sci Rep. 2018;8:1603 Available from: http://www.nature.com/articles/s41598-017-16703-9. Ivanova VT, Ivanova MV, Spitsyn BV, Garina KO, Trushakova SV, Manykin AA, et al. Interaction of nanodiamonds materials with influenza viruses. J Phys Conf Ser. 2012;345:012019 Available from: http://stacks.iop.org/1742-6596/345/i=1/a=012019?key=crossref.d83b2b535cc4ca7bf683a33aa6646d80. Pham NB, Ho TT, Nguyen GT, Le TT, Le NT, Chang H-C, et al. Nanodiamond enhances immune responses in mice against recombinant HA/H7N9 protein. J Nanobiotechnol. 2017;15:69 Available from: http://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-017-0305-2. Zitzmann C, Kaderali L. Mathematical analysis of viral replication dynamics and antiviral treatment strategies: from basic models to age-based multi-scale modeling. Front Microbiol. 2018;9 Available from: https://www.frontiersin.org/article/10.3389/fmicb.2018.01546/full. Niewiadomska AM, Jayabalasingham B, Seidman JC, Willem L, Grenfell B, Spiro D, et al. Population-level mathematical modeling of antimicrobial resistance: a systematic review. BMC Med. 2019;17:81 Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-019-1314-9. Jiang S, Wang K, Li C, Hong G, Zhang X, Shan M, et al. Mathematical models for devising the optimal Ebola virus disease eradication. J Transl Med. 2017;15:124 Available from: http://translational-medicine.biomedcentral.com/articles/10.1186/s12967-017-1224-6. Chen T-M, Rui J, Wang Q-P, Zhao Z-Y, Cui J-A, Yin L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect Dis Poverty. 2020;9:24 Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-020-00640-3.References
Author contribution statement
We are responsible in total for the contents of this paper, from inception to its completion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chakravarty, M., Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. and Transl. Res. 11, 748–787 (2021). https://doi.org/10.1007/s13346-020-00818-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00818-0