Abstract
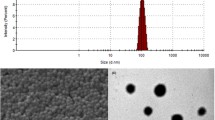
Polybutylcyanoacrylate nanoparticles (PBCA NPs) are candidates for a drug delivery system, which can cross the blood–brain barrier (BBB). Because little is known about their toxicity, we exposed cells to PBCA NPs in vitro and in vivo and monitored their life and death assays. PBCA NPs were fabricated with different surfactants according to the mini-emulsion technique. Viabilities of HeLa and HEK293 cells after NP incubation were quantified by analysing cellular metabolic activity (MTT-test). We then repetitively injected i.v. rhodamine-labelled PBCA NP variations into rats and monitored the survival and morphology of retrogradely labelled neurons by in vivo confocal neuroimaging (ICON) for five weeks. To test for carrier-efficacy and safety, PBCA NPs loaded with Kyotorphin were injected in rats, and a hot plate test was used to quantify analgesic effects. In vitro, we found dose-dependent cell death which was, however, only detectable at very high doses and mainly seen in the cultures incubated with NPs fabricated with the tensids SDS and Tween. However, the in vivo experiments did not show any NP-induced neuronal death, even with particles which were toxic at high dose in vitro, i.e. NPs with Tween and SDS. The increased pain threshold at the hot plate test demonstrated that PBCA NPs are able to cross the BBB and thus comprise a useful tool for drug delivery into the central nervous system (CNS). Our findings showing that different nanoparticle formulations are non-toxic have important implications for the value of NP engineering approaches in medicine.









Similar content being viewed by others
References
Ai J, Biazar E, Jafarpour M, Montazeri M, Majdi A, Aminifard S, Zafari M, Akbari HR, Rad HG (2011) Nanotoxicology and nanoparticle safety in biomedical designs. Int J Nanomed 6:1117–1127
Alyautdin RN, Gothier D, Petrov VE, Kharkevich DA, Kreuter J (1995) Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butylcyanoacrylate) nanoparticles. Eur J Pharm Biopharm 41:44–48
Couvreur P, Kante B, Grislain L, Roland M, Speiser P (1982) Toxicity of polyalkylcyanoacrylate nanoparticles. I. Doxorubicin-loaded nanoparticles. J Pharm Sci 71:790–792
De Jong WH, Borm PJ (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 3:133–149
Dhawan A, Sharma V (2010) Toxicity assessment of nanomaterials: methods and challenges. Anal Bioanal Chem 398:589–605
Elsaesser A, Howard CV (2012) Toxicology of nanoparticles. Adv Drug Deliv Rev 64:129–137
Fröhlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine 7:5577–5591
Gabathuler R (2010) Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol Disease 37:48–57
Henrich-Noack P, Prilloff S, Voigt N, ** J, Hintz W, Tomas J, Sabel BA (2012) In vivo visualisation of nanoparticle entry into the central nervous system tissue. Arch Toxicol 86:1099–1105
Henrich-Noack P, Voigt N, Prilloff P, Fedorov A, Sabel BA (2013) Transcorneal electrical stimulation alters morphology and survival of retinal ganglion cell after axonal damage. Neurosci Lett 543:1–6
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro 19:975–983
Kante B, Couvreur P, Dubois-Krack G, De Meester C, Guiot P, Roland M, Mercier M, Speiser P (1982) Toxicity of polyalkylcyanoacrylate nanoparticles. J Pharm Sci 71:786–790
Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA (1995) Passage of peptides through the blood–brain barrier with colloidal polymer particles (nanoparticles). Brain Res 674:171–174
Kreuter J, Petrov VE, Kharkevich DA, Alyautdin RN (1997) Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. J Control Release 49:81–87
Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R (2002) Apolipoprotein mediated transport of nanoparticle-bound drugs across the blood–brain barrier. J Drug Target 10:317–325
Ku S, Yan F, Wang Y, Sun Y, Yang N, Ye L (2010) The blood–brain barrier penetration and distribution of PEGylated fluorescein-doped magnetic silica nanoparticles in rat brain. Biochem Biophys Res Commun 394:871–876
Lam CW, James JT, McCluskey R, Hunter RL (2004) Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci 77:126–134
Love SA, Maurer-Jones MA, Thompson JW, Lin YS, Haynes CL (2012) Assessing nanoparticle toxicity. Annu Rev Anal Chem 5:181–205
Nunes A, Al-Jamal KT, Kostarelos K (2012) Therapeutics, imaging and toxicity of nanomaterials in the central nervous system. J. Control Release 161:290–306
Nyström AN, Fadeel B (2012) Safety assessment of nanomaterials: implications for nanomedicine. J. Control Release 161:403–408
Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C (2004) Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16:437–445
Prilloff S, Fan J, Henrich-Noack P, Sabel BA (2010) In vivo confocal neuroimaging (ICON): non-invasive, functional imaging of the mammalian CNS with cellular resolution. Eur J Neurosci 31:521–528
Sabel BA, Engelmann R, Humphrey MM (1997) In vivo confocal neuroimaging of CNS neurons (ICON). Nat Med 3:244–247
Sautter J, Sabel BA (1993) Recovery of Brightness discrimination in adult rats despite progressive loss of retrogradely labelled retinal ganglion cells after controlled optic nerve crush. Eur J Neurosci 5:680–690
Sautter J, Schwartz M, Duvdevani R, Sabel BA (1991) GM1 ganglioside treatment reduces visual deficits after graded crush of the rat optic nerve. Brain Res 565:23–33
Schroeder U, Sabel BA (1996) Nanoparticles, a drug carrier system to pass the blood–brain barrier, permit central analgetic effects of i.v. dalargin injections. Brain Res 710:121–124
Schroeder U, Sommerfeld P, Ulrich S, Sabel BA (1998) Nanoparticle technology for delivery of drugs across the blood–brain-barrier. Eur J Pharm Sci 87:1305–1307
Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR): Modified Opinion (after public consultation) (2006) on the appropriateness of existing methodologies to assess the potential risk associated with engineered as adventitious products of nanotechnologies. European Commission Health and Consumer Protection Directorate—General, Directorate C—Public Health and Risk Management, C7—Risk assessment, 10th Plenary Meeting, 10th of March 2006
Sharma A, Madhunapantula SV, Robertson GP (2012) Toxicological considerations when creating nanoparticle based drugs and drug delivery systems? Expert Opin Drug Metab Toxicol 8:47–69
Steuer H, Jaworski A, Stoll D, Schlosshauer B (2004) In vitro model of the outer blood-retina barrier. Brain Res Protoc 13:26–36
Steuer H, Jaworski A, Elger B, Kaussmann M, Keldenich J, Schneider H, Stoll D, Schlosshauer B (2005) Functional characterization and comparison of the outer blood–retina barrier and the blood–brain barrier. Invest Ophthalmol Vis Sci 46:1047–1053
Weiss CK, Ziener U, Landfester K (2007) A route to nonfunctionalized and functionalized poly-(n-butylcyanoacrylate) nanoparticles: preparation in miniemulsion. Macromolecules 40:928–938
Zhao J, Castranova V (2011) Toxicology of nanomaterials used in nanomedicine. J Toxicol Environ Health Part B 14:593–632
Acknowledgments
The authors thank Uta Werner for excellent technical assistance. Nadine Voigt was supported by a grant from Otto-von-Guericke University. Petra Henrich-Noack received funding from the Sybille Assmus-Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voigt, N., Henrich-Noack, P., Kockentiedt, S. et al. Toxicity of polymeric nanoparticles in vivo and in vitro. J Nanopart Res 16, 2379 (2014). https://doi.org/10.1007/s11051-014-2379-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2379-1