Abstract
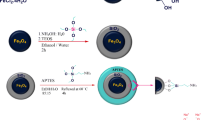
Five kinds of amino-functionalized (polyaniline, poly(1,2-diaminobenzene), poly(1,3-diaminobenzene), poly(diphenylamine), and poly(o-toluidine)) Fe3O4/SiO2 submicron composites (SCs) were prepared. The SEM and TEM results showed that these SCs possessed a sphere-like core/shell structure with an average diameter of ~500 nm. The XRD results indicated good crystallinity of Fe3O4 core, the amorphous SiO2, and amino-functionalized shells. The XPS results confirmed that amino groups were plentiful rich outside the surface of these SCs which acted as the effective groups for adsorbing the metal ions. These SCs showed a good thermal stability at 20–250 °C. The high saturation magnetization of 60–70 emu/g is better than other similar reports. In3+ adsorption coefficients from aqueous solution by these SCs were higher than 106 mL/g, indicating the higher selectivity and affinity to In3+ compared with Cd2+ and Hg2+ ions. In addition, these SCs could be magnetically reclaimed within 30 s and regenerated with acid after adsorption. The adsorption capabilities only decreased by 6 % after five cycles. The present work indicates that the amino-functionalized Fe3O4/SiO2 SCs are promising for removal of In3+ ions in field application.






Similar content being viewed by others
References
Wu N, Wei HH, Zhang LZ (2012) Efficient removal of heavy metal ions with biopolymer template synthesized mesoporous Titania beads of hundreds of micrometers size. Environ Sci Technol 46:419–425
Zhang L, Wang YN, Yuan Z, Zhao ZY, Guo XJ, Chang HC (2007) Thermodynamics of rare-scattered element In3+ adsorption by nanometer titanium oxide. Inorg Mater 43:156–161
Chang XJ, Guo YT, Wang S, Zhao R, Yang D (2004) Synthesis and efficiency of poly(acryl-p-nitrophenylamidrazone-p-nitrophenylhydrazide) chelating fibre for pre-concentration and separation of trace concentrations of Bi3+, In3+, Sn4+, Ga3+ and Ti4+. Microchim Acta 146:61–65
Lin CF, Chang KS, Tsay CW, Lee DY, Lo SL, Yasunaga T (1997) Adsorption mechanism of gallium(III) and indium(III) onto gamma-Al2O3. J Colloid Interface Sci 188:201–208
Chang WC (1993) Indium ion removal and recovery by means of water-insoluble amphoteric starches. Angew Makromol Chem 213:81–92
Gao BJ, Jiang PF, Lei HB (2006) Studies on adsorption property of novel composite adsorption material PEI/SiO2 for uric acid. Mater Lett 60:3398–3404
Wang JH, Zheng SR, Shao Y, Liu JL, Xu ZY, Zhu DQ (2010) Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349:293–299
Gao BJ, Jiang GM, An FQ (2012) Preparation of iminodiacetic acid-type composite chelating material IDAA-PGMA/SiO2 and preliminary studies on adsorption behavior of heavy metal ions and rare earth ions. J Appl Polym Sci 125:2529–2538
Yao YJ, Miao SD, Yu SM, Ma LP, Sun HQ, Wang SB (2012) Fabrication of Fe3O4/SiO2 core/shell nanoparticles attached to graphene oxide and its use as an adsorbent. J Colloid Interface Sci 379:20–26
Zhang N, Peng HY, Hu B (2012) Light-induced pH change and its application to solid phase extraction of trace heavy metals by high-magnetization Fe3O4@SiO2@TiO2 nanoparticles followed by inductively coupled plasma mass spectrometry detection. Talanta 94:278–283
Asakura R, Isobe T (2013) Surface modification of YAG:Ce3+ nanoparticles by poly(acrylic acid) and their biological application. J Mater Sci 48:8228–8234. doi:10.1007/s10853-013-7634-9
Olad A, Nabavi R (2007) Application of polyaniline for the reduction of toxic Cr(VI) in water. J Hazard Mater 147:845–851
Kumar PA, Chakraborty S (2009) Fixed-bed column study for hexavalent chromium removal and recovery by short-chain polyaniline synthesized on jute fiber. J Hazard Mater 162:1086–1098
Mao H, Liu XC, Chao DM, Cui LL, Li YX, Zhang WJ et al (2010) Preparation of unique PEDOT nanorods with a couple of cuspate tips by reverse interfacial polymerization and their electrocatalytic application to detect nitrite. J Mater Chem 20:10277–10284
Zhang LJ, Wan MX (2002) Synthesis and characterization of self-assembled polyaniline nanotubes doped with D-10-camphorsulfonic acid. Nanotechnology 13:750–755
Reddy KR, Lee KP, Kim JY, Lee Y (2008) Self-assembly and graft polymerization route to monodispersed Fe3O4@SiO2-polyniline core–shell composite nanoparticles: physical properties. J Nanosci Nanotechnol 8:5632–5639
Zhang F, Lan J, Zhao ZS, Yang Y, Tan RQ, Song WJ (2012) Removal of heavy metal ions from aqueous solution using Fe3O4-SiO2-poly(1,2-diaminobenzene) core–shell sub-micron particles. J Colloid Interface Sci 387:205–212
Fu WY, Yang HB, Liu SK, Li MH, Zou GT (2006) Preparation and magnetic characterization of core–shell structure stainless steel/silica nanoparticles. Mater Lett 60:1728–1732
Cheng ZP, Chu XZ, Yin JZ, Zhong H, Xu JM (2012) Surfactantless synthesis of Fe3O4 magnetic nanobelts by a simple hydrothermal process. Mater Lett 75:172–174
Xu YH, Zhou Y, Ma WH, Wang SX, Li SY (2013) Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles for sensitive detection and removal of Hg2+. J Nanopart Res 15:1716
Ghosh S, Badruddoza AZM, Uddin MS, Hidajat K (2011) Adsorption of chiral aromatic amino acids onto carboxymethyl-beta-cyclodextrin bonded Fe3O4/SiO2 core–shell nanoparticles. J Colloid Interface Sci 354:483–492
Jaber J, Mohsen E (2013) Synthesis of Fe3O4@silica/poly(N-isopropylacrylamide) as a novel thermo-responsive system for controlled release of H3PMo12O40 nano drug in AC magnetic field. Colloids Surf B 102:265–272
Imroz Ali AM, Mayes AG (2010) Preparation of polymeric core-shell and multilayer nanoparticles: surface-initiated polymerization using in situ synthesized photoiniferters. Macromolecules 43:837–844
Takaoka K, Otsuka T, Naka K, Niwa A, Suzuki T, Bureau C et al (2002) Analysis of X-ray photoelectron spectra of electrochemically prepared polyaniline by DFT calculations using model molecules. J Mol Struct 608:175–182
Tan S, Belanger D (2005) Characterization and transport properties of Nafion/polyaniline composite membranes. J Phys Chem B 109:23480–23490
Lei ZL, Li YL, Wei XY (2008) A facile two-step modifying process for preparation of poly(SStNa)-grafted Fe3O4/SiO2 particles. J Solid State Chem 181:480–486
Correa-Duarte MA, Giersig M, Kotov NA, Liz-Marzán LM (1998) Control of packing order of self-assembled monolayers of magnetite nanoparticles with and without SiO2 coating by microwave irradiation. Langmuir 14:6430–6435
Zhao YG, Shen HY, Pan SD, Hu MQ (2010) Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr(VI) in wastewater. J Hazard Mater 182:295–302
Fryxell GE, Lin YH, Fiskum S (2005) Actinide sequestration using self-assembled monolayers on mesoporous supports. Environ Sci Technol 39:1324–1331
Manos MJ, Petkov VG, Kanatzidis MG (2009) H2x Mn x Sn3−x S6 (x = 0.11–0.25): a novel reusable sorbent for highly specific mercury capture under extreme pH conditions. Adv Funct Mater 19:1087–1092
Acknowledgements
This work was financially supported by the Youth Science and technology innovation fund from Nan**g Agricultural University and the Youth Fund of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, F., Shi, Y., Zhao, Z. et al. Amino-functionalized Fe3O4/SiO2 magnetic submicron composites and In3+ ion adsorption properties. J Mater Sci 49, 3478–3483 (2014). https://doi.org/10.1007/s10853-014-8060-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8060-3