Abstract
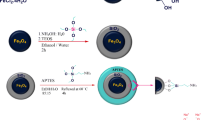
A novel magnetic core–shell nano-adsorbent, Fe3O4@SiO2/Schiff base, with Fe3O4 spheres as the core and silica Schiff base as the shell, was prepared. Schiff base in this work is a water soluble Schiff base. The surfaces of magnetite nano-particles were successfully modified by the suitable deposition of silica onto the nano-particle surface. Next, nano-composite surface was functionalized with –NH2 groups by 3-amino propyl triethoxy silane. In the next step, a water-soluble aldehyde (sodium salicylaldehyde-5-sulfonate) was synthesized and characterized. Water-soluble schiff-base supported on the coated magnetite was synthesized by the condensation reaction between the –NH2 groups of functionalized MNPs and carbonyl group of aldehyde. Nano-composite was characterized by Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, vibrating sample magnetometry and Thermo-gravimetric analysis. This nano-composite showed a high adsorption capacity for the removal of Pb(II) and Cu(II) metal ions from the aqueous solutions. The experimental data for the adsorption of Pb(II) and Cu(II) were found to follow the Langmuir isotherm and the maximum adsorption capacity were obtained 142.86 mg g−1 (0.69 mmol g−1) and 55.56 mg g−1 (0.87 mmol g−1) for pb(II) and Cu(II) metal ions respectively at pH 5. Noticeably, because the surface of MNPs has been functionalized by a water-soluble Schiff base, the nano-adsorbent dispersed easily without using the shaker or ultrasonic.










Similar content being viewed by others
References
Y.S. Al-Degs, M.I. El-Barghouthi, A.A. Issa, M.A. Khraisheh, Water Res. 40, 2645 (2006)
O. Moradi, K. Zare, Fullerenes Nanotubes Carbon Nanostruct 19, 628 (2011)
H. Needleman, Annu. Rev. Med. 55, 209 (2004)
R. Brown, B. Hingerty, J. Dewan, A. Klug, Nature 303, 543 (1983)
J. Markovac, G.W. Goldstein, Nature 334, 71 (1988)
T. Simons Neurotoxicol. Teratol. 14, 77 (1993)
D. Holtzman, C. DeVries, H. Nguyen, J. Olson, K. Bensch, Teratology 5, 97 (1984)
Y. Liu, Z. Lou, Y. Sun, X. Zhou, S.A. Baig, X. Xu, Microporous Mesoporous Mater. 246, 1 (2017)
T.M. Salama, I.O. .Ali, M.F. Bakr, S.M. El-Sheikh, M.H. Fodial, J. Inorg. Organomet. Polym. (2017). https://doi.org/10.1007/s10904-017-0704-8
T. Phuengprasop, J. Sittiwong, F. Unob, J. Hazard. Mater. 186, 502 (2011)
C. Quintelas, Z. Rocha, B. Silva, B. Fonseca, H. Figueiredo, T. Tavares, Chem. Eng. J. 149, 319 (2009)
U. Skyllberg, A. Drott, Environ. Sci. Technol. 44, 1254 (2010)
N.P. Cheremisinoff, Handbook of water and wastewater treatment technologies (Butterworth-Heinemann, Oxford, 2002)
R. Wang, X. Shi, H. Wang, C. Lei, J. Chem. Eng. Data 60, 1454 (2015)
H.-T. Fan, X.-T. Sun, Z.-G. Zhang, W.-X. Li, J. Chem. Eng. Data 59, 2106 (2014)
M.E. Mahmoud, M.M. Osman, O.F. Hafez, E. Elmelegy, J. Hazard. Mater. 173, 349 (2010)
M.M. Rao, A. Ramesh, G.P.C. Rao, K. Seshaiah, J. Hazard. Mater. 129, 123 (2006)
D.C. Culita, C.M. Simonescu, R.E. Patescu, S. Preda, N. Stanica, C. Munteanu, O. Oprea, J. Inorg. Organomet. Polym. 27, 490 (2017)
Y. Liu, R. Fu, Y. Sun, X. Zhou, S.A. Baig, X. Xu, Appl. Surf. Sci. 369, 267 (2016)
S.-H. Huang, D.-H. Chen, J. Hazard. Mater. 163, 174 (2009)
D.L. H.Li, H.HeR. **ao, P.L. Lin, Zuao, Trans. Nonferrous Met. Soc. China 23, 2657 (2013)
T. Madrakian, A. Afkhami, N.M.Ahmadi Rezvani-jalal, J. Iran. Chem. Soc. 1, 489 (2014)
A. Mehdinia, M. Jebeliyan, T. Baradaran Kayyal, A. Jabbari, Michrochim. Acta 184, 707 (2017)
A. Hethnawi, N.N. Nassar, G. Itale, Colloids Surf. A 525, 20 (2017)
H. Lee, M.K. Yu, S. Park, S. Moon, J.J. Min, Y.Y. Jeong, H.-W. Kang, S. Jon, J. Am. Chem. Soc. 129, 12739 (2007)
D.K. Kim, M. Mikhaylova, F.H. Wang, J. Kehr, B. Bjelke, Y. Zhang, T. Tsakalakos, M. Muhammed, Chem. Mater. 15, 4343 (2003)
M.H. Mashhadizadeh, M. Pesteh, M. Talakesh, I. Sheikhshoaie, M.M. Ardakani, M.A. Karimi, Spectrochim. Acta B 63, 885 (2008)
A. Fisher, D. Kara, S.J. Hill, Analyst 131, 1232 (2006)
N. Kocak, M. Sahin, G. Arslan, H.I. Ucan, J. Inorg. Organomet. Polym. 22, 166 (2012)
H. Bagheri, A. Afkhami, M. Saber-Tehrani, H. Khoshsafar, Talanta 97, 87 (2012)
A. Afkhami, T. Madrakian, R. Ahmadi, H. Bagheri, M. Tabatabaee, Microchim. Acta 175, 69 (2011)
D. Kara, A. Fisher, S.J. Hill, J. Hazard. Mater. 165, 1165 (2009)
F. Nemati, M.M. Heravi, R.S. Rad, Chin. J. Catal. 33, 1825 (2012)
K. Kim, S.S. Kim, Y. Choa, H.T. Kim, J. Ind. Eng. Chem. 13, 1137 (2007)
Y. Deng, D. Qi, C. Deng, X. Zhang, D. Zhao, J. Am. Chem. Soc. 130, 28 (2008)
M. Jafarzadeh, E. Soleimani, P. Norouzi, R. Adnan, H. Sepahvand, J. Fluor. Chem. 178, 219 (2015)
M. Botsivali, D.F. Evans, P.H. Missen, M.W. Upton, J. Chem. Soc. Dalton Trans. 1147 (1985)
R. Driouich, T. Takayanagi, M. Oshima, S. Motomizu, J. Chromatorgr. A 934, 95 (2001)
L. Chen, Z. Xu, H. Dai, S. Zhang, J. Alloys Compd. 497, 221 (2010)
Z.P. Dong, X. Tian, Y.Z. Chen, Y.P. Guo, J.T. Ma, RSC Adv. 3, 1082 (2013)
M. Yamaura, R.L. Camilo, L.C. Sampaio, M.A. Macêdo, M. Nakamurad, H.E. Tomad, J. Magn. Magn. Mater. 279, 210 (2004)
Y.L. Zhang, W.J. Ruan, X.J. Zhao, H.G. Wang, Z.A. Zhu, Polyhedron 22, 1535 (2003)
P. Hu, S. Zhang, H. Wang, D. Pan, J. Tian, Z. Tang, J. Alloys Compd. 509, 2316 (2011)
B. Issa, I.M. Obaidat, B.A. Albiss, Y. Haik, Int. J. Mol. Sci. 14, 21266 (2013)
C.P. Huang, Y.C. Chung, M.R. Liou, J. Hazard. Mater. 45, 265 (1996)
M.-M. F.Ge, B.-X. Li, Ye,, Zhao, J. Hazard. Mater. 211–212, 366–372 (2012)
F. An, B. Gao, X. Dai, J. Hazard. Mater. 192, 956 (2011)
O. Ozay, S. Ekici, Y. Baran, N. Aktas, N. Sahiner, Water Res. 43, 4403 (2009)
Acknowledgements
The authors are grateful to University of Kashan for supporting this work by Grant no. 592362.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Setoodehkhah, M., Momeni, S. Water Soluble Schiff Base Functinalized Fe3O4 Magnetic Nano-Particles as a Novel Adsorbent for the Removal of Pb(II) and Cu(II) Metal Ions from Aqueous Solutions. J Inorg Organomet Polym 28, 1098–1106 (2018). https://doi.org/10.1007/s10904-017-0770-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0770-y