Abstract
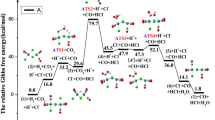
A density functional theory calculation has been carried out to investigate the mechanism of W(CO)6 and W2(CO)10 catalyzed water-gas-shift reaction (WGSR). The calculations indicate that the bimetallic catalyst (W2(CO)10) would be likely to be more highly active than the mononuclear metal-based catalyst (W(CO)6) due to the possibility of metal–metal cooperativity in reducing the barriers for the WGSR. The energetic span model is a tool to compute catalytic turnover frequencies (TOFs) which is the traditional measure of the efficiency of a catalyst. The one with the highest efficiency usually gives the highest TOF. The bimetallic catalyst (W2(CO)10) exhibits high catalytic activity towards WGSR due to the highest value of the calculated TOF (3.62 × 10−12 s−1, gas phase; 8.74 × 10−15 s−1, solvent phase), which is higher than the value of TOF (8.96 × 10−20 s−1, gas phase; 3.96 × 10−19 s−1, solvent phase) proposed by Kuriakose et al. (Inorg Chem 51:377–385, 2012). Our results will be important for designing a better catalyst for the industrially important reaction.













Similar content being viewed by others
References
Oetjen HF, Schmidt VM, Stimming U, Trila F (1996) J Electrochem Soc 143:3838–3842
Lane KR, Lee RE, Sallans L, Squires RR (1984) J Am Chem Soc 106:5768–5772
Sunderlin LS, Squires RR (1993) J Am Chem Soc 115:331–343
Torrent M, Solà M, Frenking G (1999) Organometallics 18:2801–2812
Barrows SE (2004) Inorg Chem 43:8236–8238
Zhang FL, Zhao L, Xu C, Chen Y (2010) Inorg Chem 49:3278–3281
Rozanska X, Vuilleumier R (2008) Inorg Chem 47:8635–8640
Chen Y, Zhang FL, Xu C, Gao JS, Zhai D, Zhao Z (2012) J Phys Chem A 116:2529–2535
Schulz H, Gorling A, Hieringer W (2013) Inorg Chem 52:4786–4794
King AD, King RB, Yang DB (1980) J Chem Soc Chem Commun 11:529–530
Kuriakose N, Kadam S, Vanka KA (2012) Inorg Chem 51:377–385
Shieh M, Lin SF, Guo YW, Hsu MH, Lai YW (2004) Organometallics 23:5182–5187
Wigginton JR, Chokshi A, Graham TW, McDonald R, Ferguson MJ, Cowie M (2005) Organometallics 24:6398–6410
Brooks A, Knox SAR, Stone FGA (1971) J Chem Soc A 3469–3471. doi:10.1039/J19710003469
Ford PC (1981) Acc Chem Res 14:37–42
Laine RM, Rinker RG, Ford PC (1977) J Am Chem Soc 99:252–253
Kerber RC, Pakkanen T (1979) Inorg Chim Acta 37:61–65
Gaüdek A, Kochel A, Buzar TS (2003) Organometallics 22:4869–4872
Majumdar M, Sinha A, Ghatak T, Patra SK, Sadhukhan N, Rahaman SMW, Bera JK (2010) Chem Eur J 16:2574–2585
Kozuch S, Shaik S (2011) Acc Chem Res 44:101–110
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, BakkenV Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A. 1. Gaussian Inc, Wallingford
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Andersson MP, Uvdal P (2005) J Phys Chem A 109:2937–2941
Ditchfield R, Hehre WJ, Pople JA (1971) J Chem Phys 54:724–728
Ishikawa Y, Kawakami K (2007) J Phys Chem A 111:9940–9944
Arnesen SV, Seip HM (1966) Acta Chem Scand 20:2711–2727
Ishikawa Y, Hackett P, Rayner DM (1988) J Phys Chem 92:3863–3869
Peng CY, Ayala PY, Schlege HB (1996) J Comput Chem 17:49–58
Jonas M, Stefan G (2014) J Phys Chem C 118:7615–7621
Rebecca S, Jens A, Stefan G (2014) J Phys Chem B 118:3431–3440
Kolja T, Alexei VA, Hilke B, Martin K (2011) J Phys Chem A 2011(115):8990–8996
Yu XL, Yu RQ (2013) Ind Eng Chem Res 52:11182–11188
Aleksandr VM, Christopher JC, Donald GT (2009) J Phys Chem B 113:6378–6396
Li YW, Shi XL, Zhang QZ, Hu JT, Chen JM, Wang WX (2014) Environ Sci Technol 48:5008–5016
Li YW, Zhang RM, Du LK, Zhang QZ, Wang WX (2016) Catal Sci Technol 2016(6):73–80
Li YW, Zhang RM, Du LK, Zhang QZ, Wang WX (2015) RSC Adv 5:66591–66597
Kozuch S, Martin JML (2011) ACS Catal 1:246–253
Gokhale AA, Dumesic JA, Mavrikakis M (2008) J Am Chem Soc 130:1402–1414
Uhe A, Kozuch S, Shaik S (2010) J Comput Chem 32:979–985
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20603021), the Natural Science Foundation of Shanxi (Grant No. 2013011009-6), the High School 131 Leading Talent Project of Shanxi, Undergraduate Training Programs for Innovation and Entrepreneurship of Shanxi Province (Grant Nos. 105088, 2015537, WL2015CXCY-SJ-01) and Graduate Project for Education and Innovation of Shanxi Province and Shanxi Normal University (SD2015CXXM-80, WL2015CXCY-YJ-18) and Teaching Reform Project of Shanxi Normal University (WL2015JGXM-YJ-13).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
An, X., Guo, L., Li, A. et al. Theoretical Study of the Water-Gas Shift Reaction Catalyzed by Tungsten Carbonyls. Catal Surv Asia 20, 109–120 (2016). https://doi.org/10.1007/s10563-016-9212-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-016-9212-z