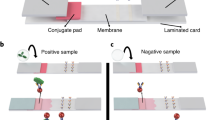
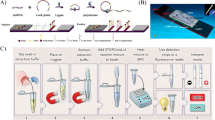
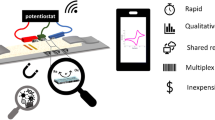
Abstract
Lateral flow assays (LFAs) have been introduced and developed over the last half century. This technology is widely used as a tool for diagnosis in several fields such as environment, food quality and healthcare. Point-of-care (POC) diagnosis using LFAs has been attracting attention of the research community, particularly aiming for the development of a platform that can evaluate of biological markers in bodily fluids such as saliva and urine. The existence of a disease or the pregnancy can be determined by a test device, before further investigation and medical treatment. LFAs make use of a disposable test strip, which can provide diagnosis result on the spot within minutes. Thus, LFAs is a promising alternative of preliminary diagnosis for laboratory instruments that are costly, time consuming and require trained personnel. This paper includes a brief overview of the conventional LFAs: material selection based on its roles and characteristics, working principles, fundamentals, applications, and design criteria. We mainly discuss the technical challenges in both engineering and biochemical aspects and recommends possible solutions. We identify current research trends and provide perspectives of advanced technologies for enhancing assay performance.









Similar content being viewed by others
References
Ahmed S, Bui M-PN, Abbas A (2016) Paper-based chemical and biological sensors: engineering aspects. Biosens Bioelectr 77:249–263
Bahadır EB, Sezgintürk MK (2016) Lateral flow assays: principles, designs and labels. TrAC Trends Anal Chem 82:286–306
Bell JM, Cameron FK (1905) The flow of liquids through capillary spaces. J Phys Chem 10(8):658–674
Benzi R, Succi S, Vergassola M (1992) The lattice Boltzmann equation: theory and applications. Phys Rep 222(3):145–197
Bogdanovic J et al (2006) Rapid detection of fungal α-amylase in the work environment with a lateral flow immunoassay. J Allergy Clin Immunol 118(5):1157–1163
Bosanquet CH (1923) LV. On the flow of liquids into capillary tubes. Lond Edinb Dublin Philos Mag J Sci 45(267):525–531
Byrnes S, Thiessen G, Fu E (2013) Progress in the development of paper-based diagnostics for low-resource point-of-care settings. Bioanalysis 5(22):2821–2836
Cao Q, Liang B, Tu T, Wei J, Fang L, Ye X (2019) Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv 9(10):5674–5681. https://doi.org/10.1039/c8ra09157a
Carrell C et al (2019) Beyond the lateral flow assay: a review of paper-based microfluidics. Microelectr Eng 206:45–54
Cate DM, Adkins JA, Mettakoonpitak J, Henry CS (2015) Recent developments in paper-based microfluidic devices. Anal Chem 87(1):19–41
Chen H, Cogswell J, Anagnostopoulos C, Faghri M (2012) A fluidic diode, valves, and a sequential-loading circuit fabricated on layered paper. Lab Chip 12(16):2909–2913. https://doi.org/10.1039/c2lc20970e
Chen X et al (2014) Development of a rapid and sensitive quantum dot-based immunochromatographic strip by double labeling PCR products for detection of Staphylococcus aureus in food. Food Control 46:225–232
Chen Y et al (2016a) Near-infrared fluorescence-based multiplex lateral flow immunoassay for the simultaneous detection of four antibiotic residue families in milk. Biosens Bioelectr 79:430–434
Chen Y et al (2016b) A dual-readout chemiluminescent-gold lateral flow test for multiplex and ultrasensitive detection of disease biomarkers in real samples. Nanoscale 8(33):15205–15212. https://doi.org/10.1039/c6nr04017a
Cho E, Mohammadifar M, Choi S (2017) A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines 8(9):265
Choi S (2016) Powering point-of-care diagnostic devices. Biotechnol Adv 34(3):321–330
Choi JR et al (2016) Polydimethylsiloxane-paper hybrid lateral flow assay for highly sensitive point-of-care nucleic acid testing. Anal Chem 88(12):6254–6264
Clark KD, Zhang C, Anderson JL (2016) Sample preparation for bioanalytical and pharmaceutical analysis. Anal Chem 88(23):11262–11270
Cummins BM, Chinthapatla R, Ligler FS, Walker GM (2017) Time-dependent model for fluid flow in porous materials with multiple pore sizes. Anal Chem 89(8):4377–4381
Dalirirad S, Steckl AJ (2019) Aptamer-based lateral flow assay for point of care cortisol detection in sweat. Sens Actuators B Chem 283:79–86
Darcy H (1856) Les Fontaines Publiques de la Ville de Dijon. Dalmont, Paris
Dharmaraja S et al (2013) Programming paper networks for point of care diagnostics. In: Microfluids, BioMEMS, and medical microsystems XI, vol 8615. International Society for Optics and Photonics. https://doi.org/10.1117/12.2006138
Di Risio S, Yan N (2007) Piezoelectric ink-jet printing of horseradish peroxidase: effect of ink viscosity modifiers on activity. Macromol Rapid Commun 28(18–19):1934–1940
Dineva MA, Candotti D, Fletcher-Brown F, Allain J-P, Lee H (2005) Simultaneous visual detection of multiple viral amplicons by dipstick assay. J Clin Microbiol 43(8):4015
Drain PK et al (2014) Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 14(3):239–249
Duan D et al (2015) Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectr 74:134–141
Dungchai W, Chailapakul O, Henry CS (2010) Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal Chim Acta 674(2):227–233
Edwards KA, Baeumner AJ (2006) Optimization of DNA-tagged dye-encapsulating liposomes for lateral-flow assays based on sandwich hybridization. Anal Bioanal Chem 386(5):1335–1343
Fang X, Wei S, Kong J (2014) Paper-based microfluidics with high resolution, cut on a glass fiber membrane for bioassays. Lab Chip 14(5):911–915. https://doi.org/10.1039/c3lc51246k
Fenton EM, Mascarenas MR, López GP, Sibbett SS (2009) Multiplex lateral-flow test strips fabricated by two-dimensional sha**. ACS Appl Mater Interfaces 1(1):124–129
Fratzl M et al (2018) Magnetic two-way valves for paper-based capillary-driven microfluidic devices. ACS Omega 3(2):2049–2057
Fridley GE, Le HQ, Fu E, Yager P (2012) Controlled release of dry reagents in porous media for tunable temporal and spatial distribution upon rehydration. Lab Chip 12(21):4321–4327. https://doi.org/10.1039/c2lc40785j
Frohnmeyer E et al (2019) Aptamer lateral flow assays for rapid and sensitive detection of cholera toxin. Analyst 144(5):1840–1849. https://doi.org/10.1039/c8an01616j
Fu E, Ramsey SA, Kauffman P, Lutz B, Yager P (2011) Transport in two-dimensional paper networks. Microfluid Nanofluidics 10(1):29–35
Fu E, Liang T, Spicar-Mihalic P, Houghtaling J, Ramachandran S, Yager P (2012) Two-dimensional paper network format that enables simple multistep assays for use in low-resource settings in the context of malaria antigen detection. Anal Chem 84(10):4574–4579
Fung K-K, Chan CP-Y, Renneberg R (2009) Development of enzyme-based bar code-style lateral-flow assay for hydrogen peroxide determination. Anal Chim Acta 634(1):89–95
Ginzbourg I, d’Humières D (1996) Local second-order boundary methods for lattice Boltzmann models. J Stat Phys 84(5):927–971
Grace W, Zaman MH (2012) Low-cost tools for diagnostic and monitoring HIV infection in low-resource settings. Bull World Health Organ 90(12):914–920
Guan L, Cao R, Tian J, McLiesh H, Garnier G, Shen W (2014) A preliminary study on the stabilization of blood ty** antibodies sorbed into paper. Cellulose 21(1):717–727
Hamraoui A, Nylander T (2002) Analytical approach for the Lucas–Washburn equation. J Colloid Interface Sci 250(2):415–421
Hamraoui A, Thuresson K, Nylander T, Eskilsson K, Yaminsky V (2001) Dynamic wetting and dewetting by aqueous solutions containing amphiphilic compounds. In: Razumas V, Lindman B, Nylander T (eds) Surface and colloid science. Progress in colloid and polymer science, vol 116. Springer, Berlin, Heidelberg, pp 113–119. https://doi.org/10.1007/3-540-44941-8_18
Hao Y et al (2014) A naphthalimide-based azo colorimetric and ratiometric probe: synthesis and its application in rapid detection of cyanide anions. Anal Methods 6(8):2478–2483. https://doi.org/10.1039/c3ay41931b
Hayes B, Murphy C, Crawley A, O’Kennedy R (2018) Developments in point-of-care diagnostic technology for cancer detection. Diagnostics 8(2):39
He JP, Katis NI, Eason WR, Sones LC (2018) Rapid multiplexed detection on lateral-flow devices using a laser direct-write technique. Biosensors 8(4):97
Hosseini S, Vázquez-Villegas P, Martínez-Chapa SO (2017) Paper and fiber-based bio-diagnostic platforms: current challenges and future needs. Appl Sci 7(8):863
Hu J et al (2013) Oligonucleotide-linked gold nanoparticle aggregates for enhanced sensitivity in lateral flow assays. Lab Chip 13(22):4352–4357. https://doi.org/10.1039/c3lc50672j
Hu J et al (2014) Advances in paper-based point-of-care diagnostics. Biosens Bioelectr 54:585–597
Hu L-M et al (2017) Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres, quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine. Biosens Bioelectr 91:95–103
Ichikawa N, Satoda Y (1994) Interface dynamics of capillary flow in a tube under negligible gravity condition. J Colloid Interface Sci 162(2):350–355
Ismail A et al (2016) Colorimetric analysis of the decomposition of S-nitrosothiols on paper-based microfluidic devices. Analyst 141(22):6314–6320. https://doi.org/10.1039/c6an01439a
Jain S et al (2015) Performance of an optimized paper-based test for rapid visual measurement of alanine aminotransferase (ALT) in fingerstick and venipuncture samples. PLoS One 10(5):e0128118
Jauset-Rubio M et al (2016) Ultrasensitive, rapid and inexpensive detection of DNA using paper based lateral flow assay. Sci Rep 6:37732
Jawaid W et al (2013) Development and validation of the first high performance-lateral flow immunoassay (HP-LFIA) for the rapid screening of domoic acid from shellfish extracts. Talanta 116:663–669
Jiang X, Fan ZH (2016) Fabrication and operation of paper-based analytical devices. Ann Rev Anal Chem 9(1):203–222
Jiang T et al (2016) Sensitive detection of Escherichia coli O157:H7 using Pt–Au bimetal nanoparticles with peroxidase-like amplification. Biosens Bioelectr 77:687–694
Juntunen E, Myyryläinen T, Salminen T, Soukka T, Pettersson K (2012) Performance of fluorescent europium(III) nanoparticles and colloidal gold reporters in lateral flow bioaffinity assay. Anal Biochem 428(1):31–38
Kasetsirikul S, Buranapong J, Srituravanich W, Kaewthamasorn M, Pimpin A (2016) The development of malaria diagnostic techniques: a review of the approaches with focus on dielectrophoretic and magnetophoretic methods. Malar J 15(1):358
Kavosi B, Hallaj R, Teymourian H, Salimi A (2014) Au nanoparticles/PAMAM dendrimer functionalized wired ethyleneamine–viologen as highly efficient interface for ultra-sensitive α-fetoprotein electrochemical immunosensor. Biosens Bioelectr 59:389–396
Khan MS et al (2010) Biosurface engineering through ink jet printing. Colloids Surf B Biointerfaces 75(2):441–447
Koo CKW, He F, Nugen SR (2013) An inkjet-printed electrowetting valve for paper-fluidic sensors. Analyst 138(17):4998–5004. https://doi.org/10.1039/C3AN01114C
Koponen A et al (1998) Permeability of three-dimensional random fiber webs. Phys Rev Lett 80(4):716–719
Kunkel HG, Tiselius A (1951) Electrophoresis of proteins on filter paper. J Gen Physiol 35(1):89–118
Lavi B, Marmur A, Bachmann J (2008) Porous media characterization by the two-liquid method: effect of dynamic contact angle and inertia. Langmuir 24(5):1918–1923
Lee J-H et al (2015) Multiplex diagnosis of viral infectious diseases (AIDS, hepatitis C, and hepatitis A) based on point of care lateral flow assay using engineered proteinticles. Biosens Bioelectr 69:213–225
Lee S, Mehta S, Erickson D (2016) Two-color lateral flow assay for multiplex detection of causative agents behind acute febrile illnesses. Anal Chem 88(17):8359–8363
Li et al (2009) Development of up-converting phosphor technology-based lateral-flow assay for rapidly quantitative detection of hepatitis B surface antibody. Diagn Microbiol Infect Dis 63(2):165–172
Li X, Tian J, Shen W (2010) Progress in patterned paper sizing for fabrication of paper-based microfluidic sensors. Cellulose 17(3):649–659
Li X et al (2012) A fast and sensitive immunoassay of avian influenza virus based on label-free quantum dot probe and lateral flow test strip. Talanta 100:1–6
Li X, Zwanenburg P, Liu X (2013) Magnetic timing valves for fluid control in paper-based microfluidics. Lab Chip 13(13):2609–2614. https://doi.org/10.1039/C3LC00006K
Liang L et al (2016) Aptamer-based fluorescent and visual biosensor for multiplexed monitoring of cancer cells in microfluidic paper-based analytical devices. Sens Actuators B Chem 229:347–354
Liu C et al (2011) Lateral flow immunochromatographic assay for sensitive pesticide detection by using Fe3O4 nanoparticle aggregates as color reagents. Anal Chem 83(17):6778–6784
Liu H, Li X, Crooks RM (2013) Paper-based SlipPAD for high-throughput chemical sensing. Anal Chem 85(9):4263–4267
Liu Z et al (2018) Liquid wicking behavior in paper-like materials: mathematical models and their emerging biomedical applications. Microfluid Nanofluidics 22(11):132
Lucas R (1918) Ueber das Zeitgesetz des kapillaren Aufstiegs von Flüssigkeiten. Kolloid-Zeitschrift 23(1):15–22
Lutz BR, Trinh P, Ball C, Fu E, Yager P (2011) Two-dimensional paper networks: programmable fluidic disconnects for multi-step processes in shaped paper. Lab Chip 11(24):4274–4278
Lutz B, Liang T, Fu E, Ramachandran S, Kauffman P, Yager P (2013) Dissolvable fluidic time delays for programming multi-step assays in instrument-free paper diagnostics. Lab Chip 13(14):2840–2847
Mandal P, Dey R, Chakraborty S (2012) Electrokinetics with “paper-and-pencil” devices. Lab Chip 12(20):4026–4028. https://doi.org/10.1039/C2LC40681K
Mao X, Ma Y, Zhang A, Zhang L, Zeng L, Liu G (2009) Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal Chem 81(4):1660–1668
Martin AJP, Synge RLM (1941) A new form of chromatogram employing two liquid phases. Biochem J 35(12):1358
Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM (2008) Simple telemedicine for develo** regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem 80(10):3699–3707
Martinez WA et al (2010) Programmable diagnostic devices made from paper and tape. Lab Chip 10(19):2499–2504. https://doi.org/10.1039/c0lc00021c
Mashamba-Thompson PT, Jama AN, Sartorius B, Drain KP, Thompson MR (2017) Implementation of point-of-care diagnostics in rural primary healthcare clinics in South Africa: perspectives of key stakeholders. Diagnostics 7(1):3
Mdluli P et al (2014) Gold nanoparticle based tuberculosis immunochromatographic assay: the quantitative ESE quanti analysis of the intensity of test and control lines. Biosens Bioelectr 54:1–6
Medina A, Pérez-Rosales C, Pineda A, Higuera FJ (2001) Imbibition in pieces of paper with different shapes. Revista Mexicana de Fisica 47:537–541
Mendez S et al (2010) Imbibition in porous membranes of complex shape: quasi-stationary flow in thin rectangular segments. Langmuir 26(2):1380–1385
Millipore (2013) Rapid lateral flow test strip: considerations for product development, Millipore Corporation
Millot G, Voisin B, Loiez C, Wallet F, Nseir S (2017) The next generation of rapid point-of-care testing identification tools for ventilator-associated pneumonia. Ann Transl Med 5(22):451
Mora MF et al (2019) Patterning and modeling three-dimensional microfluidic devices fabricated on a single sheet of paper. Anal Chem 91(13):8298–8303
Morales-Narváez E, Naghdi T, Zor E, Merkoçi A (2015) Photoluminescent lateral-flow immunoassay revealed by graphene oxide: highly sensitive paper-based pathogen detection. Anal Chem 87(16):8573–8577
Morbioli GG, Mazzu-Nascimento T, Stockton AM, Carrilho E (2017) Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs)—a review. Anal Chim Acta 970:1–22
Mu X et al (2015) A paper-based skin patch for the diagnostic screening of cystic fibrosis. Chem Commun 51(29):6365–6368. https://doi.org/10.1039/c5cc00717h
Nakhal RS, Wood D, Woodhouse C, Creighton SM (2012) False-positive pregnancy tests following enterocystoplasty. BJOG Int J Obstet Gynaecol 119(3):366–368
Nguyen N-T, Wu Z (2004) Micromixers—a review. J Micromech Microeng 15(2):R1–R16
Noh H, Phillips ST (2010) Fluidic timers for time-dependent, point-of-care assays on paper. Anal Chem 82(19):8071–8078
O’Farrell B (2015) Lateral flow technology for field-based applications—basics and advanced developments. Topics Companion Anim Med 30(4):139–147
O’Keeffe M et al (2003) Preliminary evaluation of a lateral flow immunoassay device for screening urine samples for the presence of sulphamethazine. J Immunol Methods 278(1):117–126
Oh YK, Joung H-A, Han HS, Suk H-J, Kim M-G (2014) A three-line lateral flow assay strip for the measurement of C-reactive protein covering a broad physiological concentration range in human sera. Biosens Bioelectr 61:285–289
Osborn JL, Lutz B, Fu E, Kauffman P, Stevens DY, Yager P (2010) Microfluidics without pumps: reinventing the T-sensor and H-filter in paper networks. Lab Chip 10(20):2659–2665. https://doi.org/10.1039/c004821f
Park J-M, Jung H-W, Chang YW, Kim H-S, Kang M-J, Pyun J-C (2015) Chemiluminescence lateral flow immunoassay based on Pt nanoparticle with peroxidase activity. Anal Chim Acta 853:360–367
Park J, Shin JH, Park J-K (2016) Pressed paper-based dipstick for detection of foodborne pathogens with multistep reactions. Anal Chem 88(7):3781–3788
Parolo C, Medina-Sánchez M, de la Escosura-Muñiz A, Merkoçi A (2013) Simple paper architecture modifications lead to enhanced sensitivity in nanoparticle based lateral flow immunoassays. Lab Chip 13(3):386–390. https://doi.org/10.1039/c2lc41144j
Qian YH, D’Humières D, Lallemand P (1992) Lattice BGK models for Navier–Stokes equation. Europhys Lett (EPL) 17(6):479–484
Qiu W et al (2015) Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosens Bioelectr 64:367–372
Report MR (2018) Lateral flow assay market by application, product, technique, end user—global forecast to 2023. [Online]. https://www.marketsandmarkets.com/Market-Reports/lateral-flow-assay-market-167205133.html. Accessed 3 July 2019
Rezk AR, Qi A, Friend JR, Li WH, Yeo LY (2012) Uniform mixing in paper-based microfluidic systems using surface acoustic waves. Lab Chip 12(4):773–779. https://doi.org/10.1039/C2LC21065G
Rideal EK (1922) CVIII. On the flow of liquids under capillary pressure. Lond Edinb Dublin Philos Mag J Sci 44(264):1152–1159
Rooz (2010). The power of paper: elegant solutions in diagnostics [online]. https://miter.mit.edu/articlepower-paper-elegant-solutions-diagnostics/. Accessed 2 July 2019
Sajid M, Kawde A-N, Daud M (2015) Designs, formats and applications of lateral flow assay: a literature review. J Saudi Chem Soc 19(6):689–705
Salminen T, Juntunen E, Khanna N, Pettersson K, Talha SM (2016) Anti-HCV immunoassays based on a multiepitope antigen and fluorescent lanthanide chelate reporters. J Virol Methods 228:67–73
Sanger F (1988) Sequences, sequences, and sequences. Ann Rev Biochem 57(1):1–29
Shen J et al (2015) Immunochromatographic assay for quantitative and sensitive detection of hepatitis B virus surface antigen using highly luminescent quantum dot-beads. Talanta 142:145–149
Shiroma LY, Santhiago M, Gobbi AL, Kubota LT (2012) Separation and electrochemical detection of paracetamol and 4-aminophenol in a paper-based microfluidic device. Anal Chim Acta 725:44–50
Sicard B et al (2015) Tools for water quality monitoring and map** using paper-based sensors and cell phones. Water Res 70:360–369
Siebold A, Nardin M, Schultz J, Walliser A, Oppliger M (2000) Effect of dynamic contact angle on capillary rise phenomena. Colloids Surf A Physicochem Eng Asp 161(1):81–87
Singer JM, Plotz CM (1956) The latex fixation test: I. Application to the serologic diagnosis of rheumatoid arthritis. Am J Med 21(6):888–892
Song S et al (2014) Multiplex lateral flow immunoassay for mycotoxin determination. Anal Chem 86(10):4995–5001
Stefano GB, Kream RM (2018) The micro-hospital: 5G telemedicine-based care. Med Sci Monit Basic Res 24:103–104
Takalkar S, Baryeh K, Liu G (2017) Fluorescent carbon nanoparticle-based lateral flow biosensor for ultrasensitive detection of DNA. Biosens Bioelectr 98:147–154
Tang R et al (2017) Improved analytical sensitivity of lateral flow assay using sponge for HBV nucleic acid detection. Sci Rep 7(1):1360
Toley BJ, McKenzie B, Liang T, Buser JR, Yager P, Fu E (2013) Tunable-delay shunts for paper microfluidic devices. Anal Chem 85(23):11545–11552
Torre LA et al (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68(4):284–296
Tsai T-T, Huang T-H, Ho NY-J, Chen Y-P, Chen C-A, Chen C-F (2019) Development of a multiplex and sensitive lateral flow immunoassay for the diagnosis of periprosthetic joint infection. Sci Rep 9(1):15679
Urteaga R, Elizalde E, Berli CLA (2018) Transverse solute dispersion in microfluidic paper-based analytical devices (μPADs). Analyst 143(10):2259–2266. https://doi.org/10.1039/c8an00149a
Wang ZL (2012) Self-powered nanosensors and nanosystems. Adv Mater 24(2):280–285
Wang Y, Xu H, Wei M, Gu H, Xu Q, Zhu W (2009) Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay. Mater Sci Eng C 29(3):714–718
Wang W, Wu W-Y, Wang W, Zhu J-J (2010) Tree-shaped paper strip for semiquantitative colorimetric detection of protein with self-calibration. J Chromatogr A 1217(24):3896–3899
Washburn EW (1921) The dynamics of capillary flow. Phys Rev 17(3):273–283
Weaver AA et al (2013) Paper analytical devices for fast field screening of beta lactam antibiotics and antituberculosis pharmaceuticals. Anal Chem 85(13):6453–6460
Wei X et al (2016) Microfluidic distance readout sweet hydrogel integrated paper-based analytical device (μDiSH-PAD) for visual quantitative point-of-care testing. Anal Chem 88(4):2345–2352
Wen H-W, Borejsza-Wysocki W, DeCory TR, Durst RA (2005) Development of a competitive liposome-based lateral flow assay for the rapid detection of the allergenic peanut protein Ara h1. Anal Bioanal Chem 382(5):1217–1226
Whelan WJ (1995) The advent of paper chromatography. FASEB J 9(2):287–288
Whitaker S (1986) Flow in porous media I: a theoretical derivation of Darcy’s law. Transp Porous Media 1(1):3–25
Wide L (1969) Early diagnosis of pregnancy. Lancet 294(7626):863–864
Wong R, Tse H (2009) Lateral flow immunoassay, 1st edn. Humana Press, NY, USA, p 224
Wu T et al (2018) Enhanced lateral flow assay with double conjugates for the detection of exosomes. Sci China Chem 61(11):1423–1429
**ao G et al (2019) A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose 26(7):4553–4562
Rivas L, Medina-Sánchez M, de la Escosura-Muñiz A, Merkoçi A (2014) Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab Chip 14(22):4406–4414. https://doi.org/10.1039/c4lc00972j
Yager P et al (2006) Microfluidic diagnostic technologies for global public health. Nature 442(7101):412–418
Yager P, Domingo GJ, Gerdes J (2018) Point-of-care diagnostics for global health. Ann Rev Biomed Eng 10(1):107–144
Yan J et al (2014) Effect of physiochemical property of Fe3O4 particle on magnetic lateral flow immunochromatographic assay. Sens Actuators B Chem 197:129–136
Yang Y, Noviana E, Nguyen MP, Geiss BJ, Dandy DS, Henry CS (2017) Paper-based microfluidic devices: emerging themes and applications. Anal Chem 89(1):71–91
Yetisen K, Akram MS, Lowe CR (2013) Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 13(12):2210–2251. https://doi.org/10.1039/c3lc50169h
Ying N et al (2017) Lateral flow nucleic acid biosensor for sensitive detection of microRNAs based on the dual amplification strategy of duplex-specific nuclease and hybridization chain reaction. PLoS One 12(9):e0185091
Yonekita T et al (2013) Development of a novel multiplex lateral flow assay using an antimicrobial peptide for the detection of Shiga toxin-producing Escherichia coli. J Microbiol Methods 93(3):251–256
Yu WW, White IM (2013) Inkjet-printed paper-based SERS dipsticks and swabs for trace chemical detection. Anal Artic 138(4):1020–1025
Zhang D et al (2018) Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens Bioelectr 106:204–211
Zhao Y et al (2016) Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci Rep 6:21342
Zhong ZW, Wu RG, Wang ZP, Tan HL (2015) An investigation of paper based microfluidic devices for size based separation and extraction applications. J Chromatogr B 1000:41–48
Zhou M (2015) Recent progress on the development of biofuel cells for self-powered electrochemical biosensing and logic biosensing: a review. Electroanalysis 27(8):1786–1810
Zweig G, Whitaker JR, Block RJ (1971) Paper chromatography and electrophoresis: paper chromatography by J. Sherman and G. Zweig (paper chromatography and electrophoresis). Academic Press, Cambridge
Acknowledgements
The authors acknowledge the support of the Australian Research Council (DP180100055) and higher degree research scholarships GUIPRS and GUPRS Scholarships to S.K. from the Griffith University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kasetsirikul, S., Shiddiky, M.J.A. & Nguyen, NT. Challenges and perspectives in the development of paper-based lateral flow assays. Microfluid Nanofluid 24, 17 (2020). https://doi.org/10.1007/s10404-020-2321-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-020-2321-z