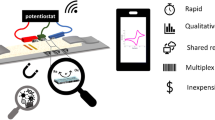
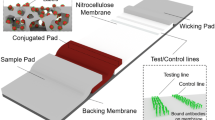
Abstract
Conventional lateral flow assay (LFA) is typically performed by observing the color changes in the test lines by naked eyes, which achieves considerable commercial success and has a significant impact on the fields of food safety, environment monitoring, disease diagnosis, and other applications. However, this qualitative detection method is not very suitable for low levels of disease biomarkers’ detection. Although many nanomaterials are used as new labels for LFA, additional readers limit their application to some extent. Fortunately, a lot of work has been done for improving the sensitivity of LFA. In this review, currently reported LFA sensitivity enhancement methods with an objective evaluation are summarized, such as sample pretreatment, the change of flow rate, and label evolution, and future development direction and challenges of LFAs are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid, portable, sensitive, and inexpensive detection of analytes from complex samples is essential for in vitro diagnostics [2]. It is estimated that improving the technique of diagnostic tests for infectious diseases in develo** countries can annually save at least 1.2 million deaths [3]. Especially when facing the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a rapid and facile screening strategy, which is employed in airports, customs, and community, shows the great significance to prevent epidemic and resume ship** and economic development.
Lateral flow assay (LFA) deployed as point-of-care testing (POCT), owing to its rapidity, simplicity, stability, and visual characteristics [4], has been vital in enabling faster diagnosis, directing medical interventions, and mitigating the transmission of infectious diseases [2].
Conventionally, the results of LFA are read out by naked eyes, by measuring the color change due to the accumulation of gold nanoparticles (AuNPs). This detection strategy is simple and rapid, and the early pregnancy test is the outstanding representative. However, these results are qualitative and lack of sensitivity and may function only for certain applications. As for the detection of critical biochemical markers present in extremely small amounts in a sample, such as myocardial infarction and cancer, these methods do not afford sufficient sensitivity, which restrict their applications. In recent years, the development of new nanomaterials has broadened the type of labels available for LFA to enhance sensitivity. These nanomaterials can be roughly divided into three categories according to the type of readout [5], that is, naked-eye detection, fluorescence detection, and non-optical readout detection. Carbon nanoparticles [6, 7], carbon nanotubes [8, 9], and dye-loaded latex beads [10, 11] can provide an alternative to AuNPs for naked-eye detection. Fluorescent labels are generally recommended for low concentrations of targets and quantitative detection. Suitable fluorescent nanoparticles include fluorescent microspheres (FMs) [12], quantum dots (QDs) [11], upconverting nanoparticles (UCNPs) [13], and liposomes with fluorescent dyes [14]. LFA with non-optical readout can be comparable with that of LFA with fluorescent labels, such as magnetic nanoparticles [15,16,17] and nanoparticles for electrochemical readings[18,19,20]. However, they either cannot provide a strong signal as AuNPs for naked-eye detection or comes with a higher cost and the need for an external reader.
In order to enhance the signal–noise ratio, a lot of signal amplification methods have been employed in POCT, such as gas-propelled [21, 22], enzyme-mimicking accelerated signal enhancement [23, 24], and cascade amplification [ Wu YH, Zhou YF, Leng YK, Lai WH, Huang XL, **ong YH (2020) Emerging design strategies for constructing multiplex lateral flow test strip sensors. Biosens Bioelectron 157:13 Soh JH, Chan HM, Ying JY (2020) Strategies for develo** sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today 30:17 Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, Rodriguez W, Bassett IV (2014) Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 14:239–249 Gervais L, De Rooij N, Delamarche E (2011) Microfluidic chips for point-of-care immunodiagnostics. Adv Mater 23:H151–H176 Parolo C, Sena-Torralba A, Bergua JF, Calucho E, Fuentes-Chust C, Hu LM, Rivas L, Alvarez-Diduk R, Nguyen EP, Cinti S, Quesada-Gonzalez D, Merkoci A (2020) Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat Protoc 15:3788–3816 Oliveira-Rodriguez M, Serrano-Pertierra E, Garcia AC, Martin SL, Mo MY, Cernuda-Morollon E, Blanco-Lopez MC (2017) Point-of-care detection of extracellular vesicles: sensitivity optimization and multiple-target detection. Biosens Bioelectron 87:38–45 Noguera P, Posthuma-Trumpie GA, Van Tuil M, Van Der Wal FJ, De Boer A, Moers A, Van Amerongen A (2011) Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of shiga toxin-producing escherichia coli. Anal Bioanal Chem 399:831–838 Qiu WW, Xu H, Takalkar S, Gurung AS, Liu B, Zheng YF, Guo ZB, Baloda M, Baryeh K, Liu GD (2015) Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosens Bioelectron 64:367–372 Yao L, Teng J, Zhu MY, Zheng L, Zhong YH, Liu GD, Xue F, Chen W (2016) Mwcnts based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens Bioelectron 85:331–336 Greenwald R, Esfandiari J, Lesellier S, Houghton R, Pollock J, Aagaard C, Andersen P, Hewinson RG, Chambers M, Lyashchenko K (2003) Improved serodetection of mycobacterium bovis infection in badgers (meles meles) using multiantigen test formats. Diagn Micr Infec Dis 46:197–203 Morales-Narvaez E, Naghdi T, Zor E, Merkoci A (2015) Photo luminescent lateral-flow immunoassay revealed by graphene oxide: highly sensitive paper-based pathogen detection. Anal Chem 87:8573–8577 Zhang J, Lv XF, Feng W, Li XQ, Li KJ, Deng YL (2018) Aptamer-based fluorometric lateral flow assay for creatine kinase mb. Microchim Acta 185:364 Kim J, Kwon JH, Jang J, Lee H, Kim S, Hahn YK, Kim SK, Lee KH, Lee S, Pyo H, Song CS, Lee J (2018) Rapid and background-free detection of avian influenza virus in opaque sample using nir-to-nir upconversion nanoparticle-based lateral flow immunoassay platform. Biosens Bioelectron 112:209–215 Edwards KA, Baeumner AJ (2009) Liposome-enhanced lateral-flow assays for the sandwich-hybridization detection of rna. Methods Mol Biol (Clifton, N.J.) 504:185–215 Panferov VG, Safenkova IV, Zherdev AV, Dzantiev BB (2017) Setting up the cut-off level of a sensitive barcode lateral flow assay with magnetic nanoparticles. Talanta 164:69–76 Lago-Cachon D, Oliveira-Rodriguez M, Rivas M, Blanco-Lopez MC, Martinez-Garcia JC, Moyano A, Salvador M, Garcia JA (2017) Scanning magneto-inductive sensor for quantitative assay of prostate-specific antigen. IEEE Magn Lett 8:5 Moyano A, Salvador M, Martinez-Garcia JC, Socoliuc V, Vekas L, Peddis D, Alvarez MA, Fernandez M, Rivas M, Blanco-Lopez MC (2019) Magnetic immunochromatographic test for histamine detection in wine. Anal Bioanal Chem 411:6615–6624 Ruiz-Vega G, Kitsara M, Pellitero MA, Baldrich E, Del Campo FJ (2017) Electrochemical lateral flow devices: towards rapid immunomagnetic assays. ChemElectroChem 4:880–889 Cinti S, Moscone D, Arduini F (2019) Preparation of paper-based devices for reagentless electrochemical (bio)sensor strips. Nat Protoc 14:2437–2451 Li ZD, Li F, ** of fully written microfluidic biosensor. Biosens Bioelectron 98:478–485 Liu XL, Wang YP, Gao YF, Song YJ (2021) Gas-propelled biosensors for quantitative analysis. Analyst 146:1115–1126 Li Y, Xuan J, Song YJ, Qi WJ, He BS, Wang P, Qin LD (2016) Nanoporous glass integrated in volumetric bar-chart chip for point-of-care diagnostics of non-small cell lung cancer. ACS Nano 10:1640–1647 Sun L, Zhao Q, Liu XL, Pan YC, Gao YF, Yang JJ, Wang YZ, Song YJ (2020) Enzyme-mimicking accelerated signal enhancement for visually multiplexed quantitation of telomerase activity. Chem Commun 56:6969–6972 Dervisevic M, Senel M, Cevik E (2017) Novel impedimetric dopamine biosensor based on boronic acid functional polythiophene modified electrodes. Mater Sci Eng C-Mater Biol Appl 72:641–649 Shen JJ, Zhou XM, Shan YY, Yue HH, Huang R, Hu JM, **ng D (2020) Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and crispr-cas13a amplification reaction. Nat Commun 11:10 Zhang WY, Hao WH, Liu XT, Sun XR, Yan JL, Wang YC (2020) Visual detection of mirnas using enzyme-free amplification reactions and ratiometric fluorescent probes. Talanta 219:6 Cheng Y-SL, Rees T, Wright J (2014) A review of research on salivary biomarkers for oral cancer detection. Clin Transl Med 3:3–3 Mukama O, Nie CR, Habimana JD, Meng XG, Ting Y, Songwe F, Al Farga A, Mugisha S, Rwibasira P, Zhang YH, Zeng LW (2020) Synergetic performance of isothermal amplification techniques and lateral flow approach for nucleic acid diagnostics. Anal Biochem 600:13 Gonzalez JM, Foley MW, Bieber NM, Bourdelle PA, Niedbala RS (2011) Development of an ultrasensitive immunochromatography test to detect nicotine metabolites in oral fluids. Anal Bioanal Chem 400:3655–3664 Niemz A, Ferguson TM, Boyle DS (2011) Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol 29:240–250 Zhang J, Cao JJ, Zhu MS, Xu MG, Shi F (2019) Loop-mediated isothermal amplification-lateral-flow dipstick (lamp-lfd) to detect mycoplasma ovipneumoniae. World J Microb Biot 35:10 Hu JQ, Wang Y, Su HJ, Ding HM, Sun XC, Gao H, Geng Y, Wang ZC (2020) Rapid analysis of escherichia coli o157:H7 using isothermal recombinase polymerase amplification combined with triple-labeled nucleotide probes. Mol Cell Probe 50:6 Mahmoudi T, De La Guardia M, Baradaran B (2020) Lateral flow assays towards point -of -care cancer detection: a review of current progress and future trends. Trac-trend Anal Chem 125:20 Luo K, Kim HY, Oh MH, Kim YR (2020) Paper-based lateral flow strip assay for the detection of foodborne pathogens: principles, applications, technological challenges and opportunities. Crit Rev Food Sci 60:157–170 Yao MD, Lv XF, Deng YL, Rasheed M (2019) Specific and simultaneous detection of micro rna 21 and let-7a by rolling circle amplification combined with lateral flow strip. Anal Chim Acta 1055:115–125 Mei XR, Zhai XW, Lei CW, Ye XL, Kang ZZ, Wu X, **ang R, Wang YL, Wang HN (2019) Development and application of a visual loop-mediated isothermal amplification combined with lateral flow dipstick (lamp-lfd) method for rapid detection of salmonella strains in food samples. Food Control 104:9–19 Li TT, Jalbani YM, Zhang GL, Zhao ZY, Wang ZY, Zhao Y, Zhao XY, Chen AL (2019) Rapid authentication of mutton products by recombinase polymerase amplification coupled with lateral flow dipsticks. Sensor Actuat B-chem 290:242–248 Niazi A, Jorjani O-N, Nikbakht H, Gill P (2013) A nanodiagnostic colorimetric assay for 18s rrna of leishmania pathogens using nucleic acid sequence-based amplification and gold nanorods. Mol Diagn Ther 17:363–370 Kolm C, Martzy R, Fuhrer M, Mach RL, Krska R, Baumgartner S, Farnleitner AH, Reischer GH (2019) Detection of a microbial source tracking marker by isothermal helicase-dependent amplification and a nucleic acid lateral-flow strip test. Sci Rep 9:9 Ying N, Ju CJ, Sun XW, Li LT, Chang HB, Song GP, Li ZY, Wan JY, Dai EY (2017) Lateral flow nucleic acid biosensor for sensitive detection of micrornas based on the dual amplification strategy of duplex-specific nuclease and hybridization chain reaction. PLoS ONE 12:12 Kuhn H, Demidov VV, Frank-Kamenetskii MD (2002) Rolling-circle amplification under topological constraints. Nucleic Acids Res 30:574–580 Zhao WA, Ali MM, Brook MA, Li YF (2008) Rolling circle amplification: applications in nanotechnology and biodetection with functional nucleic acids. Angew Chem Int Edit 47:6330–6337 Ali MM, Li F, Zhang ZQ, Zhang KX, Kang DK, Ankrum JA, Le XC, Zhao WA (2014) Rolling circle amplification: a versatile tool for chemical biology, materials science and medicine. Chem Soc Rev 43:3324–3341 Kor K, Turner APF, Zarei K, Atabati M, Beni V, Mak WC (2016) Structurally responsive oligonucleotide-based single-probe lateral-flow test for detection of mirna-21 mimics. Anal Bioanal Chem 408:1475–1485 Zhang CY, Chen GF, Wang YY, Zhou J, Li CH (2019) Establishment and application of hyperbranched rolling circle amplification coupled with lateral flow dipstick for the sensitive detection of karenia mikimotoi. Harmful Algae 84:151–160 Liu FG, Chen GF, Zhang CY, Wang YY, Zhou J (2019) Exponential rolling circle amplification coupled with lateral flow dipstick strips as a rapid and sensitive method for the field detection of karlodinium veneficum. J Appl Phycol 31:2423–2436 Kim TY, Lim MC, Woo MA, Jun BH (2018) Radial flow assay using gold nanoparticles and rolling circle amplification to detect mercuric ions. Nanomaterials 8:13 Liu M, Hui CY, Zhang Q, Gu J, Kannan B, Jahanshahi-Anbuhi S, Filipe CDM, Brennan JD, Li YF (2016) Target-induced and equipment-free DNA amplification with a simple paper device. Angew Chem Int Edit 55:2709–2713 Hui CY, Liu M, Li YF, Brennan JD (2018) A paper sensor printed with multifunctional bio/nano materials. Angew Chem Int Edit 57:4549–4553 Wu LD, Ma C, Zheng XX, Liu HY, Yu JH (2015) Paper-based electrochemiluminescence origami device for protein detection using assembled cascade DNA-carbon dots nanotags based on rolling circle amplification. Biosens Bioelectron 68:413–420 Dou MW, Dominguez DC, Li XJ, Sanchez J, Scott G (2014) A versatile pdms/paper hybrid microfluidic platform for sensitive infectious disease diagnosis. Anal Chem 86:7978–7986 Joung J, Ladha A, Saito M, Kim N-G, Woolley AE, Segel M, Barretto RPJ, Ranu A, Macrae RK, Faure G, Ioannidi EI, Krajeski RN, Bruneau R, Huang M-LW, Yu XG, Li JZ, Walker BD, Hung DT, Greninger AL, Jerome KR, Gootenberg JS, Abudayyeh OO, Zhang F (2020) Detection of sars-cov-2 with sherlock one-pot testing. New Engl J Med 383:1492 Piepenburg O, Williams CH, Stemple DL, Armes NA (2006) DNA detection using recombination proteins. PLoS Biol 4:1115–1121 Zasada AA, Zacharczuk K, Forminska K, Wiatrzyk A, Ziolkowski R, Malinowska E (2018) Isothermal DNA amplification combined with lateral flow dipsticks for detection of biothreat agents. Anal Biochem 560:60–66 Karakkat BB, Hockemeyer K, Franchett M, Olson M, Mullenberg C, Koch PL (2018) Detection of root-infecting fungi on cool-season turfgrasses using loop-mediated isothermal amplification and recombinase polymerase amplification. J Microbiol Meth 151:90–98 Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F (2019) Sherlock: nucleic acid detection with crispr nucleases. Nat Protoc 14:2986–3012 Fu MQ, Chen GF, Zhang CY, Wang YY, Sun R, Zhou J (2019) Rapid and sensitive detection method for karlodinium veneficum by recombinase polymerase amplification coupled with lateral flow dipstick. Harmful Algae 84:1–9 Saxena A, Pal V, Tripathi NK, Goel AK (2019) Development of a rapid and sensitive recombinase polymerase amplification-lateral flow assay for detection of burkholderia mallei. Transbound Emerg Dis 66:1016–1022 Sun N, Wang Y, Yao XY, Chen FF, Gao DY, Wang WP, Li XJ (2019) Visual signal generation for the detection of influenza viruses by duplex recombinase polymerase amplification with lateral flow dipsticks. Anal Bioanal Chem 411:3591–3602 Du XJ, Zang YX, Liu HB, Li P, Wang S (2018) Recombinase polymerase amplification combined with lateral flow strip for listeria monocytogenes detection in food. J Food Sci 83:1041–1047 Xu YC, Wei YJ, Cheng N, Huang KL, Wang WR, Zhang L, Xu WT, Luo YB (2018) Nucleic acid biosensor synthesis of an all-in-one universal blocking linker recombinase polymerase amplification with a peptide nucleic acid-based lateral flow device for ultrasensitive detection of food pathogens. Anal Chem 90:708–715 Choi JR, Hu J, Gong Y, Feng SS, WaW A, **guan-Murphy B, Xu F (2016) An integrated lateral flow assay for effective DNA amplification and detection at the point of care. Analyst 141:2930–2939 Seok Y, Joung HA, Byun JY, Jeon HS, Shin SJ, Kim S, Shin YB, Han HS, Kim MG (2017) A paper-based device for performing loop-mediated isothermal amplification with real-time simultaneous detection of multiple DNA targets. Theranostics 7:2220–2230 Xu GL, Nolder D, Reboud J, Oguike MC, Van Schalkwyk DA, Sutherland CJ, Cooper JM (2016) Paper-origami-based multiplexed malaria diagnostics from whole blood. Angew Chem Int Edit 55:15250–15253 Zuo P, Li XJ, Dominguez DC, Ye BC (2013) A pdms/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 13:3921–3928 Fang XE, Chen H, Xu LJ, Jiang XY, Wu WJ, Kong JL (2012) A portable and integrated nucleic acid amplification microfluidic chip for identifying bacteria. Lab Chip 12:1495–1499 Safavieh M, Ahmed MU, Sokullu E, Ng A, Braescuac L, Zourob M (2014) A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst 139:482–487 Connelly JT, Rolland JP, Whitesides GM (2015) “Paper machine” for molecular diagnostics. Anal Chem 87:7595–7601 Chen YT, Cheng N, Xu YC, Huang KL, Luo YB, Xu WT (2016) Point-of-care and visual detection of p. Aeruginosa and its toxin genes by multiple lamp and lateral flow nucleic acid biosensor. Biosens Bioelectron 81:317–323 Zhu X, Wang X, Han L, Chen T, Wang L, Li H, Li S, He L, Fu X, Chen S, **ng M, Chen H, Wang Y (2020) Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of covid-19. Biosens Bioelectron 166. Kim C, Yoo YK, Il Han S, Lee J, Lee D, Lee K, Hwang KS, Lee KH, Chung S, Lee JH (2017) Battery operated preconcentration-assisted lateral flow assay. Lab Chip 17:2451–2458 Moghadam BY, Connelly KT, Posner JD (2015) Two orders of magnitude improvement in detection limit of lateral flow assays using lsotachophoresis. Anal Chem 87:1009–1017 Chiu RYT, Jue E, Yip AT, Berg AR, Wang SJ, Kivnick AR, Nguyen PT, Kamei DT (2014) Simultaneous concentration and detection of biomarkers on paper. Lab Chip 14:3021–3028 Tang RH, Yang H, Choi JR, Gong Y, Hu J, Feng SS, **guan-Murphy B, Mei QB, Xu F (2016) Improved sensitivity of lateral flow assay using paper-based sample concentration technique. Talanta 152:269–276 Mashayekhi F, Chiu RYT, Le AM, Chao FC, Wu BM, Kamei DT (2010) Enhancing the lateral-flow immunoassay for viral detection using an aqueous two-phase micellar system. Anal Bioanal Chem 398:2955–2961 Mashayekhi F, Le AM, Nafisi PM, Wu BM, Kamei DT (2012) Enhancing the lateral-flow immunoassay for detection of proteins using an aqueous two-phase micellar system. Anal Bioanal Chem 404:2057–2066 Chen F, Ming X, Chen XX, Gan M, Wang BG, Xu F, Wei H (2014) Immunochromatographic strip for rapid detection of cronobacter in powdered infant formula in combination with silica-coated magnetic nanoparticles separation and 16s rrna probe. Biosens Bioelectron 61:306–313 Fang ZY, Wu W, Lu XW, Zeng LW (2014) Lateral flow biosensor for DNA extraction-free detection of salmonella based on aptamer mediated strand displacement. Amplification Biosens Bioelectron 56:192–197 Wang DB, Tian B, Zhang ZP, Wang XY, Fleming J, Bi LJ, Yang RF, Zhang XE (2015) Detection of bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “road closure.” Biosens Bioelectron 67:608–614 Lu XW, Liang XL, Dong JH, Fang ZY, Zeng LW (2016) Lateral flow biosensor for multiplex detection of nitrofuran metabolites based on functionalized magnetic beads. Anal Bioanal Chem 408:6703–6709 Zhang B, Ma WJ, Li FX, Gao WC, Zhao Q, Peng WP, Piao JF, Wu XL, Wang HJ, Gong XQ, Chang J (2017) Fluorescence quenching-based signal amplification on immunochromatography test strips for dual-mode sensing of two biomarkers of breast cancer. Nanoscale 9:18711–18722 Bahadir EB, Sezginturk MK (2016) Lateral flow assays: principles, designs and labels. Trac-trend Anal Chem 82:286–306 Kumar R, Singh CK, Kamle S, Sinha RP, Bhatnagar RK, Kachru DN (2010) Development of nanocolloidal gold based immunochromatographic assay for rapid detection of transgenic vegetative insecticidal protein in genetically modified crops. Food Chem 122:1298–1303 Blazkova M, Mickova-Holubova B, Rauch P, Fukal L (2009) Immunochromatographic colloidal carbon-based assay for detection of methiocarb in surface water. Biosens Bioelectron 25:753–758 Zeng Q, Mao X, Xu H, Wang S, Liu G (2009) Quantitative immunochromatographic strip biosensor for the detection of carcinoembryonic antigen tumor biomarker in human plasma. Rivas L, Medina-Sanchez M, De La Escosura-Muniz A, Merkoci A (2014) Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab Chip 14:4406–4414 Choi JR, Liu Z, Hu J, Tang RH, Gong Y, Feng SS, Ren H, Wen T, Yang H, Qu ZG, **guan-Murphy B, Xu F (2016) Polydimethylsiloxane-paper hybrid lateral flow assay for highly sensitive point-of-care nucleic acid testing. Anal Chem 88:6254–6264 Choi JR, Yong KW, Tang RH, Gong Y, Wen T, Yang H, Li A, Chia YC, **guan-Murphy B, Xu F (2017) Lateral flow assay based on paper-hydrogel hybrid material for sensitive point-of-care detection of dengue virus. Adv Healthc Mater 6:9 Xu ZG, Zhao Y, Dai LM, Lin T (2014) Multi-responsive janus liquid marbles: The effect of temperature and acidic/basic vapors. Part Part Syst Char 31:839–842 Parolo C, De La Escosura-Muniz A, Merkoci A (2013) Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens Bioelectron 40:412–416 Choi JR, Hu J, Feng SS, WaW A, **guan-Murphy B, Xu F (2016) Sensitive biomolecule detection in lateral flow assay with a portable temperature-humidity control device. Biosens Bioelectron 79:98–107 Katis IN, He PJW, Eason RW, Sones CL (2018) Improved sensitivity and limit-of-detection of lateral flow devices using spatial constrictions of the flow-path. Biosens Bioelectron 113:95–100 Urusov AE, Petrakova AV, Kuzmin PG, Zherdev AV, Sveshnikov PG, Shafeev GA, Dzantiev BB (2015) Application of gold nanoparticles produced by laser ablation for immunochromatographic assay labeling. Anal Biochem 491:65–71 Yao YY, Guo WS, Zhang J, Wu YD, Fu WH, Liu TT, Wu XL, Wang HJ, Gong XQ, Liang XJ, Chang J (2016) Reverse fluorescence enhancement and colorimetric bimodal signal readout immunochromatography test strip for ultrasensitive large-scale screening and postoperative monitoring. ACS Appl Mater Interfaces 8:22963–22970 Kong MM, Yang B, Gong CJ, Wang H, Li X, Zhao KS, Li JJ, Wu F, Liu X, Hu Z (2017) Development of immunochromatographic colloidal gold test strip for rapid detection of haemophilus influenzae in clinical specimens. J Appl Microbiol 123:287–294 Choi DH, Lee SK, Oh YK, Bae BW, Lee SD, Kim S, Shin YB, Kim MG (2010) A dual gold nanoparticle conjugate-based lateral flow assay (lfa) method for the analysis of troponin i. Biosens Bioelectron 25:1999–2002 Posthuma-Trumpie GA, Korf J, Van Amerongen A (2009) Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem 393:569–582 Zhong YH, Chen YJ, Yao L, Zhao DP, Zheng L, Liu GD, Ye YW, Chen W (2016) Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchim Acta 183:1989–1994 Mei ZL, Qu W, Deng Y, Chu HQ, Cao JX, Xue F, Zheng L, El-Nezamic HS, Wu YC, Chen W (2013) One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol a (bpa) in aqueous samples. Biosens Bioelectron 49:457–461 Zhu MY, Wang Y, Deng Y, Yao L, Adeloju SB, Pan DD, Xue F, Wu YC, Zheng L, Chen W (2014) Ultrasensitive detection of mercury with a novel one-step signal amplified lateral flow strip based on gold nanoparticle-labeled ssdna recognition and enhancement probes. Biosens Bioelectron 61:14–20 Serebrennikova K, Samsonova J, Osipov A (2018) Hierarchical nanogold labels to improve the sensitivity of lateral flow immunoassay. Nano-Micro Lett 10:8 Kim W, Lee S, Jeon S (2018) Enhanced sensitivity of lateral flow immunoassays by using water-soluble nanofibers and silver-enhancement reactions. Sensor Actuat B-chem 273:1323–1327 Anfossi L, Di Nardo F, Giovannoli C, Passini C, Baggiani C (2013) Increased sensitivity of lateral flow immunoassay for ochratoxin a through silver enhancement. Anal Bioanal Chem 405:9859–9867 Cho IH, Bhunia A, Irudayaraj J (2015) Rapid pathogen detection by lateral-flow immunochromatographic assay with gold nanoparticle-assisted enzyme signal amplification. Int J Food Microbiol 206:60–66 Li JW, Baird MA, Davis MA, Tai WY, Zweifel LS, Waldorf KMA, Gale M, Rajagopal L, Pierce RH, Gao XH (2017) Dramatic enhancement of the detection limits of bioassays via ultrafast deposition of polydopamine. Nat Biomed Eng 1:12 Panferov VG, Safenkova IV, Varitsev YA, Drenova NV, Kornev KP, Zherdev AV, Dzantiev BB (2016) Development of the sensitive lateral flow immunoassay with silver enhancement for the detection of ralstonia solanacearum in potato tubers. Talanta 152:521–530 Rodriguez MO, Covian LB, Garcia AC, Blanco-Lopez MC (2016) Silver and gold enhancement methods for lateral flow immunoassays. Talanta 148:272–278 Yang W, Li XB, Liu GW, Zhang BB, Zhang Y, Kong T, Tang JJ, Li DN, Wang Z (2011) A colloidal gold probe-based silver enhancement immunochromatographic assay for the rapid detection of abrin-a. Biosens Bioelectron 26:3710–3713 Anfossi L, Giovannoli C, Giraudi G, Biagioli F, Passini C, Baggiani C (2012) A lateral flow immunoassay for the rapid detection of ochratoxin a in wine and grape must. J Agr Food Chem 60:11491–11497 Ambrosi A, Castaneda MT, Killard AJ, Smyth MR, Alegret S, Merkoci A (2007) Double-codified gold nanolabels for enhanced immunoanalysis. Anal Chem 79:5232–5240 Tang DP, Yuan R, Chal YQ (2008) Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal Chem 80:1582–1588 Lai GS, Yan F, Ju HX (2009) Dual signal amplification of glucose oxidase-functionalized nanocomposites as a trace label for ultrasensitive simultaneous multiplexed electrochemical detection of tumor markers. Anal Chem 81:9730–9736 Li J, Song SP, Li D, Su Y, Huang Q, Zhao Y, Fan CH (2009) Multi-functional crosslinked au nanoaggregates for the amplified optical DNA detection. Biosens Bioelectron 24:3311–3315 He YQ, Zhang SQ, Zhang XB, Baloda M, Gurung AS, Xu H, Zhang XJ, Liu GD (2011) Ultrasensitive nucleic acid biosensor based on enzyme-gold nanoparticle dual label and lateral flow strip biosensor. Biosens Bioelectron 26:2018–2024 Mao X, Ma YQ, Zhang AG, Zhang LR, Zeng LW, Liu GD (2009) Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal Chem 81:1660–1668 Jia XF, Wang CW, Rong Z, Li J, Wang KL, Qie ZW, **ao R, Wang SQ (2018) Dual dye-loaded au@ag coupled to a lateral flow immunoassay for the accurate and sensitive detection of mycoplasma pneumoniae infection. RSC Adv 8:21243–21251 Blanco-Covian L, Montes-Garcia V, Girard A, Fernandez-Abedul MT, Perez-Juste J, Pastoriza-Santos I, Faulds K, Graham D, Blanco-Lopez MC (2017) Au@ag serrs tags coupled to a lateral flow immunoassay for the sensitive detection of pneumolysin. Nanoscale 9:2051–2058 Cima-Cabal MD, Mendez FJ, Vazquez F, Garcia-Suarez MD, De Los Toyos JR (2001) A specific and ultrasensitive chemiluminescent sandwich ELISA test for the detection and quantitation of pneumolysin. J Immunoass Immunoch 22:99–112 Bastus NG, Comenge J, Puntes V (2011) Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening. Langmuir 27:11098–11105 Wang R, Kim K, Choi N, Wang X, Lee J, Jeon JH, Rhie GE, Choo J (2018) Highly sensitive detection of high-risk bacterial pathogens using sers-based lateral flow assay strips. Sensor Actuat B-chem 270:72–79 Zhang D, Huang L, Liu B, Ni HB, Sun LD, Su EB, Chen HY, Gu ZZ, Zhao XW (2018) Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell sers nanotags. Biosens Bioelectron 106:204–211 Xu QF, Xu H, Gu HC, Li JB, Wang YY, Wei M (2009) Development of lateral flow immunoassay system based on superparamagnetic nanobeads as labels for rapid quantitative detection of cardiac troponin i. Mat Sci Eng C-bio S 29:702–707 Zhu JM, Zou NL, Zhu DN, Wang J, ** QH, Zhao JL, Mao HJ (2011) Simultaneous detection of high-sensitivity cardiac troponin i and myoglobin by modified sandwich lateral flow immunoassay: proof of principle. Clin Chem 57:1732–1738 Zhang D, Huang L, Liu B, Su EB, Chen HY, Gu ZZ, Zhao XW (2018) Quantitative detection of multiplex cardiac biomarkers with encoded sers nanotags on a single t line in lateral flow assay. Sensor Actuat B-chem 277:502–509 Hwang J, Lee S, Choo J (2016) Application of a sers-based lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin b. Nanoscale 8:11418–11425 Gao XF, Zheng P, Kasani S, Wu S, Yang F, Lewis S, Nayeem S, Engler-Chiurazzi EB, Wigginton JG, Simpkins JW, Wu NQ (2017) Paper-based surface-enhanced raman scattering lateral flow strip for detection of neuron-specific enolase in blood plasma. Anal Chem 89:10104–10110 North SH, Shriver-Lake LC, Taitt CR, Ligler FS, Rapid analytical methods for on-site triage for traumatic brain injury, in: R.G. Cooks,E.S. Yeung (Eds.) Annual review of analytical chemistry, vol 5, Annual Reviews, Palo Alto, 2012, pp. 35–56. Li SJ, Zhang Y, Wen WJ, Sheng W, Wang JY, Wang S, Wang JP (2019) A high-sensitivity thermal analysis immunochromatographic sensor based on au nanoparticle-enhanced two-dimensional black phosphorus photothermal-sensing materials. Biosens Bioelectron 133:223–229 Qin ZP, Chan WCW, Boulware DR, Akkin T, Butler EK, Bischof JC (2012) Significantly improved analytical sensitivity of lateral flow immunoassays by using thermal contrast. Angew Chem Int Edit 51:4358–4361 Song S, Choi S, Ryu S, Kim S, Kim T, Shin J, Jung HI, Joo C (2018) Highly sensitive paper-based immunoassay using photothermal laser speckle imaging. Biosens Bioelectron 117:385–391 Wang YR, Qin ZP, Boulware DR, Pritt BS, Sloan LM, Gonzalez IJ, Bell D, Rees-Channer RR, Chiodini P, Chan WCW, Bischof JC (2016) Thermal contrast amplification reader yielding 8-fold analytical improvement for disease detection with lateral flow assays. Anal Chem 88:11774–11782 Ge XX, Asiri AM, Du D, Wen W, Wang SF, Lin YH (2014) Nanomaterial-enhanced paper-based biosensors. Trac-trend. Anal Chem 58:31–39 Nguyen VT, Song S, Park S, Joo C (2020) Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens Bioelectron 152:17 Zhao YF, Huang Y, Zhao XW, Mcclelland JF, Lu M (2016) Nanoparticle-based photoacoustic analysis for highly sensitive lateral flow assays. Nanoscale 8:19204–19210 Park C, Kwon EY, Shin NY, Choi SM, Kim SH, Park SH, Lee DG, Choi JH, Yoo JH (2011) Evaluation of nucleic acid sequence based amplification using fluorescence resonance energy transfer (fret-nasba) in quantitative detection of aspergillus 18s rrna. Med Mycol 49:73–79 Sharma A, Tok AIY, Lee C, Ganapathy R, Palaniappan A, Liedberg B (2019) Magnetic field assisted preconcentration of biomolecules for lateral flow assaying. Sensor Actuat B-chem 285:431–437 Ren W, Mohammed SI, Wereley S, Irudayaraj J (2019) Magnetic focus lateral flow sensor for detection of cervical cancer biomarkers. Anal Chem 91:2876–2884 Fu X, Cheng Z, Yu J, Choo P, Chen L, Choo J (2016) A sers-based lateral flow assay biosensor for highly sensitive detection of hiv-1 DNA. Biosens Bioelectron 78:530–537 Choi S, Hwang J, Lee S, Lim DW, Joo H, Choo J (2017) Quantitative analysis of thyroid-stimulating hormone (tsh) using sers-based lateral flow immunoassay. Sensor Actuat B-chem 240:358–364References
Acknowledgements
This work was financially supported by the Space Medical Experiment Project of China Manned Space Program (HYZHXM04003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Y., Jiang, H., Li, X. et al. Recent advances in sensitivity enhancement for lateral flow assay. Microchim Acta 188, 379 (2021). https://doi.org/10.1007/s00604-021-05037-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05037-z