Abstract
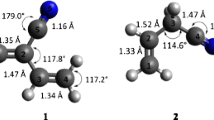
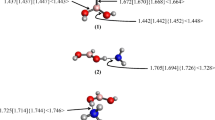
Derivatives of 5,6-diborylbicyclo[2.1.1]hexane have been presented as the novel bicyclic diborane receptors for the recognition of fluoride ion. The MP2/6-311+G**//B3LYP/6-311+G** calculated results suggest for much higher fluoride ion affinity for these studied receptors. Dicyano derivative of 5,6-diborylbicyclo[2.1.1]hexane (7) shows ~25.0 kcal/mol higher fluoride ion affinity than the prototype 1,8-naphthalenediylbis(dimethylborane) (1). Further, it has been shown that the affinity of these receptors can be tuned through remote substituent effect. The molecular electrostatic isopotential surface calculations reveal the change in the value of V S,max on boron center due to remote substitutions. Quantum theory of atoms in molecule analyses shows that the binding of F− to the boron centers of the receptor molecules is non-covalent in nature. Incorporation of chromogenic units at the remote positions also influences the affinity of receptors toward analytes. Further, the calculated higher fluoride ion affinities of these receptors in the aqueous medium suggest that they can be promising candidates to function as F− ion receptors in water medium also. The Cl− and Br− ion affinities of these receptors have also been discussed. The designed bicyclo[2.1.1]hexane receptors are synthetically achievable as similar systems have been reported (Wiberg et al. in J Am Chem Soc 83:3998, 1961; Martínez et al. in Tetrahedron Asym 4:2333, 1993).










Similar content being viewed by others
References
Jouvin MH, De Vernejoul MC, Druet P (1987) Am J Kidney Dis 10:136–139
Wade CR, Broomsgrove AEJ, Aldridge S, Gabbai FP (2010) Chem Rev 110:3958–3984 and references therein
Gale PA (2010) Chem Soc Rev 39:3746–3771 and references therein
Cametti M, Rissanen K (2009) Chem Commun 2809–2829 and references therein
Zhang M, Li M, Li F, Cheng Y, Zhang J, Yi T, Huang C (2008) Dyes Pigm 77:408–414
Werner F, Schneider H-J (2000) Helv Chim Acta 83:465–478
Gale PA, Camiolo S, Chapman CP, Light ME, Hursthouse MB (2001) Tetrahedron Lett 42:5095–5097
Dudič M, Lhoták P, Stibor I, Lang K, Prošková P (2003) Org Lett 5:149–152
Bucher C, Zimmerman RS, Lynch V, Sessler JL (2001) J Am Chem Soc 123:9716–9717
Woods CJ, Camiolo S, Light ME, Coles SJ, Hursthouse MB, King MA, Gale PA, Essex JW (2002) J Am Chem Soc 124:8644–8652
Sasaki S-i, Mizuno M, Naemura K, Tobe Y (2000) J Org Chem 65:275–283
Galbraith E, James TD (2010) Chem Soc Rev 39:3831–3842 and references therein
Guo Z, Shin I, Yoon J (2012) Chem Commun 48:5956–5967
Katz HE (1985) J Org Chem 50:5027–5032
Katz HE (1986) J Am Chem Soc 108:7640–7645
Dusmund C, Sandanayake K, Shinkai S (1995) J Chem Soc Chem Commun 333–334
Bresner C, Aldridge S, Fallis I, Jones AC, Ooi L-L (2005) Angew Chem Int Ed 44:3606–3609
Day JK, Bresner C, Coombs ND, Fallis IA, Ooi L-L, Aldridge S (2008) Inorg Chem 47:793–804
Kubo Y, Yamamoto M, Ikeda M, Takeuchi M, Shinkai S, Yamaguchi S, Tamao K (2003) Angew Chem Int Ed 42:2036–2040
Timoshkin AY, Frenking G (2008) Organometallics 27:371–380
Melaïmi M, Sole S, Chiu C-W, Wang H, Gabbaï FP (2006) Inorg Chem 45:8136–8143
Veltheer JE, Burger P, Bergman RG (1995) J Am Chem Soc 117:12478–12488
Krossing I, Raabe I (2004) Chem Eur J 10:5017–5030
Bresner C, Haynes C, Addy DA, Broomsgrove AEJ, Fitzpatrick P, Vidovic D, Thompson AL, Fallis IA, Aldridge S (2010) New J Chem 34:1652–1659
Huh JO, Kim H, Lee KM, Lee YS, Do Y, Lee MH (2010) Chem Commun 46:1138–1140
Solé S, Gabbaï FP (2004) Chem Commun 1284–1285
Henderson LD, Piers WE, Irvine GJ, McDonald R (2002) Organometallics 21:340–345
Metz MV, Schwartz DJ, Stern CL, Marks TJ, Nickias PN (2002) Organometallics 21:4159–4168
Emslie DJM, Piers WE, Parvez M (2003) Angew Chem Int Ed 42:1252–1255
Gabbaï FP (2003) Angew Chem Int Ed 42:2218–2221
Lewis SP, Taylor NJ, Piers WE, Collins S (2003) J Am Chem Soc 125:14686–14687
Morrison DJ, Piers WE, Parvez M (2004) Synlett 13:2429–2433
Melaimi M, Gabbaï FP (2005) Adv Organomet Chem 53:61–69
Venkatasubbaiah K, Zakharov LN, Kassel WS, Rheingold AL, Jäkle F (2005) Angew Chem Int Ed 44:5428–5433
Chase PA, Henderson LD, Piers WE, Parvez M, Clegg W, Elsegood MRJ (2006) Organometallics 25:349–357
Venkatasubbaiah K, Nowil I, Herber RH, Jäkle F (2007) Chem Commun 2154–2156
Dorsey CL, Jewula P, Hudnall TW, Hoefelmeyer JD, Taylor TJ, Honesty NR, Chiu C-W, Schulte M, Gabbaï FP (2008) Dalton Trans 4442–4450
Kawachi A, Tani A, Shimada J, Yamamoto Y (2008) J Am Chem Soc 130:4222–4223
Boshra R, Venkatasubbaiah K, Doshi A, Lalancette RA, Kakalis L, Jäkle F (2007) Inorg Chem 46:10174–10186
Melaïmi M, Gabbaï FP (2005) J Am Chem Soc 127:9680–9681
Lee MH, Gabbaï FP (2007) Inorg Chem 46:8132–8138
Kim Y, Hudnall TW, Bouhadir G, Bourissou D, Gabbaï FP (2009) Chem Commun 3729–3731
Hudnall TW, Kim Y-M, Bebbington MWP, Bourissou D, Gabbaï FP (2008) J Am Chem Soc 130:10890–10891
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1988) Ab initio molecular orbital theory. Wiley, New York
Møller C, Plesset MS (1934) Phys Rev 46:618–621
Cancès E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041
Mennucci B, Tomasi J (1997) J Chem Phys 106:5151–5158
Barone V, Cossi M, Tomasi J (1997) J Chem Phys 107:3210–3221
Barone V, Cossi M, Tomasi J (1998) J Comput Chem 19:404–417
Tomasi J, Mennucci B, Cancès E (1999) J Mol Struct (Theochem) 464:211–226
Bader RFW (1990) Atoms in molecule: a quantum theory. Oxford University Press, New York
Politzer P, Murray JS (1991) In: Lipkowitz KB, Boyd DB (eds) Reviews in computational chemistry, vol 2, ch 7. VCH Publishers, New York
Tomasi J, Bonaccorsi R, Cammi R (1990). In: Maksic R (ed) Theoretical models of chemical bonding. Springer, Berlin
Pathak RK, Gadre SR (1990) J Chem Phys 93:1770–1773
Scrocco E, Tomasi J (1979) Adv Quantum Chem 11:115–193
Murray JS, Politzer P (1998) J Mol Struct (Theochem) 425:107–114
Brinck T, Murray JS, Politzer P (1992) Mol Phys 76:609–617
Murray JS, Politzer P (1988) Chem Phys Lett 152:364–370
Haeberlein M, Murray JS, Brinck T, Politzer P (1992) Can J Chem 70:2209–2214
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679–1691
Politzer P, Murray JS, Bulat FP (2010) J Mol Model 16:1731–1742
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T Jr, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision B01. Gaussian, Inc., Wallingford, CT
MarVin, version 5.9.4; ChemAxon: Budapest, Hungary, (2007) http://www.chemaxon.com
Evers EC, Freitag WO, Kriner WA, MacDiarmid AG (1959) J Am Chem Soc 81:5106–5108
Wiberg KB, Lowry BR, Colby TH (1961) J Am Chem Soc 83:3998–4006
Martínez AG, Vilar ET, Barcina JO, Herrero Sdlm, Cerero MER, Hanack M, Subramanian M (1993) Tetrahedron Asymmetry 4:2333–2334
Shankar R, Kolandaivel P, Senthilkumar L (2011) J Phys Org Chem 24:553–567
Roberts JAS, Chen M-C, Seyam AM, Li L, Zuccaccia C, Stahl NG, Marks TJ (2007) J Am Chem Soc 129:12713–12733
Singh A, Ganguly B (2007) J Phys Chem A 111:6468–6471
Murray JS, Lane P, Clark T, Riley KE, Politzer P (2012) J Mol Model 18:541–548
Duke R, McCabe M, Schmitt WT, Gunnlaugsson T (2012) J Org Chem 77:3115–3126
Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM (2006) Coord Chem Rev 250:3094–3117
Sangster J (1997) Octanol-water partition coefficients: fundamentals and physical chemistry, vol 2 of Wiley series in solution chemistry. Wiley, Chichester
Tiwari YB, Miller MM, Wasik SP, Martier DE (1982) J Chem Eng Data 27:451–454
Acknowledgments
MKK and DS are thankful to UGC, New Delhi, India, and BG thanks CSIR & DST, New Delhi, India, for financial support. Authors are also thankful to PAULI HPC Cluster facilities available at NCL, Pune, India. We thank the reviewer for valuable suggestions to improve the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kesharwani, M.K., Sahu, D., Desai, K. et al. In silico studies toward the recognition of fluoride ion by novel bicyclic diborane receptors and tuning through remote substituent effects. Theor Chem Acc 132, 1358 (2013). https://doi.org/10.1007/s00214-013-1358-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-013-1358-4