Search
Search Results
-
Gene Therapy
The gene with the expression vector is inserted in the organ, which needs to be repaired. The gene is expressed locally in the area and recombinant...
-
Regulatory Aspects of Gene Therapy and Cell Therapy Products A Global Perspective
This book discusses the different regulatory pathways for Advanced Therapy Medicinal Products implemented by national agencies in North and South...

-
Cardiac Gene Therapy Methods and Protocols
This second edition volume expands on the previous edition with updated techniques and discussions on topics such as gene suppression, editing, and...
-
Biomanufacturing Aspects of Gene Therapy
The notion that genetic material could be transferred to a host patient for therapeutic benefit has been around for over half a century. However, it...
-
European Pharmacopoeia’s Approach to Cell and Gene Therapy: Focus on How Gene Therapy Texts Are Evolving
The European Pharmacopoeia (Ph. Eur.) constitutes single recognised common standard for the quality control of medicines in Europe, which is also...
-
International Harmonization for Cell and Gene Therapy Products
To increase the global availability of cell and gene therapy products, international regulatory agencies engage in programs that enhance dialogue...
-
Updates on Cardiac Gene Therapy Research and Methods: Overview of Cardiac Gene Therapy
Gene therapy has made a significant progress in clinical translation over the past few years with several gene therapy products currently approved or...
-
The Regulation of Cell Therapy and Gene Therapy Products in Switzerland
This chapter describes the regulation of cell and gene therapy products (CGTPs) in Switzerland and its legal basis. The Swiss Agency for Therapeutic...
-
Viral Vectors in Gene Replacement Therapy
AbstractThroughout the years, several hundred million people with rare genetic disorders have been receiving only symptom management therapy....

-
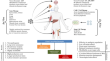
Recent developments in gene therapy research in India
Inherited genetic disorders are progressive in nature and lead to organ dysfunction or death in severe cases. At present, there are no permanent...
-
Gene-edited cells: novel allogeneic gene/cell therapy for epidermolysis bullosa
Epidermolysis bullosa (EB) is a group of rare genetic skin fragility disorders, which are hereditary. These disorders are associated with mutations...

-
Suppressor tRNA in gene therapy
Suppressor tRNAs are engineered or naturally occurring transfer RNA molecules that have shown promise in gene therapy for diseases caused by nonsense...
-
Current Insights into the Potential of Gene Therapy to Treat Rare Mitochondrial Diseases
Mitochondrial disorders represent a rare category of diseases that arise from abnormalities in essential proteins necessary for the proper...
-
Revolutionizing cancer care strategies: immunotherapy, gene therapy, and molecular targeted therapy
Despite the availability of technological advances in traditional anti-cancer therapies, there is a need for more precise and targeted cancer...

-
Gene Therapy and Cell Therapy: Bioanalytical Challenges and Practical Solutions
Clinical development of gene therapy (GTx) and cell therapy (CTx) modalities involve extensive and challenging immunogenicity and pharmacokinetic...
-
Regulatory Aspects of Cell and Gene Therapy Products: The Japanese Perspective
Regulations for regenerative medicine for human use, such as cell and gene therapy (CGT), have evolved in accordance with advancements in clinical...
-
Cancer Treatment with Ferroptosis by a Combination of Iron Nanoparticles and Gene Therapy
Despite advancements in cancer treatment, the efficacy of current clinical therapies is still limited. Ferroptosis, an iron-dependent form of...
-
Definitive Treatments for Chronic Granulomatous Disease with a Focus on Gene Therapy
The past 20 years have witnessed massive progress in the early diagnosis of Chronic Granulomatous Disease (CGD), leading to prompt initiation of...
-
Gene editing and therapy in acquired and inherited cardiovascular disorders
Gene editing and therapy holds immense promise in addressing cardiovascular disorders by enabling precise modifications to the genetic groundworks of...

-
Base Editors-Mediated Gene Therapy in Hematopoietic Stem Cells for Hematologic Diseases
Base editors, developed from the CRISPR/Cas system, consist of components such as deaminase and Cas variants. Since their emergence in 2016, the...

