Abstract
Altered metabolism of lipids is currently considered a hallmark characteristic of many malignancies, including colorectal cancer (CRC). Lipids are a large group of metabolites that differ in terms of their fatty acid composition. This review summarizes recent evidence, documenting many alterations in the content and composition of fatty acids, polar lipids, oxylipins and triacylglycerols in CRC patients’ sera, tumor tissues and adipose tissue. Some of altered lipid molecules may be potential biomarkers of CRC risk, development and progression. Owing to a significant role of many lipids in cancer cell metabolism, some of lipid metabolism pathways may also constitute specific targets for anti-CRC therapy.
Similar content being viewed by others
Introduction
Finding a disease, the course of which is not related to lipid alterations can be challenging. Lipids raise a growing interest as potential biomarkers in many clinical conditions. This highlights the importance of lipidomic studies in understanding, diagnosing and treating numerous human pathologies, among them cancer; the use of lipidomics could create an opportunity to design targeted therapies, prognostic or screening biomarkers [1]. In everyday clinical practice, lipid status is estimated based on serum concentrations of total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL) and triacylglycerols (TGs). While only a limited information can be obtained from the analysis of those lipid fractions, other currently available techniques, e.g. mass spectrometry, may provide a detailed insight into the structure and function of some specific lipid species. In this review paper, we discuss lipid alterations associated with colorectal cancer (CRC), with special emphasis on fatty acids (FAs) and their potential therapeutic and diagnostic applications in patients with this malignancy.
Most cancers found in the colon or rectum are adenocarcinomas arising from pathological lesions in the epithelial cells of colorectal mucosa [2]. Vast majority of CRCs are thought to evolve from conventional adenomas through as a result of several dozens of mutations; this process is referred to as the adenoma-to-carcinoma sequence [3]. Most CRCs are sporadic malignancies and are not associated with inherited mutations in established cancer-related genes [4]. However, about 20–30% CRC may be associated with inherited mutations [5]. A progressive accumulation of multiple genetic mutations contributes to transition from normal mucosa to benign adenoma, severe dysplasia, and eventually, a frank carcinoma. It is estimated that approximately 15% of sporadic colon cancers are a consequence of malfunction in mismatch repair genes, whereas other 80–85% are associated with mutations in adenomatous polyposis coli (APC) gene. Furthermore, colon cancer may develop as a consequence of inflammatory bowel disease, on a different, yet uncharacterized pathway. Malignant transformation requires further genetic alterations [6]. Less than 50% of colon cancers harbor mutated KRAS, a protein that is involved in intracellular signal transduction [7, 8]. Approximately 50% of colonic lesions with high-grade dysplasia and about 75% of frank cancers may carry p53 mutations [6, 7]. A neoplastic disease cannot be effectively managed without the understanding of distinctive characteristics of cancer cells that contribute to tumor development. One of them is enhanced proliferation [9]. Two main genetic defects found in CRC, KRAS and p53 mutations, are both associated with enhanced proliferation [10, 11]. Intensively proliferating cancer cells display some unique metabolic patterns due to which they may obtain enough energy for new biomass synthesis. Cancer cells have a unique ability to generate energy in a nutrient-deficient environment. Since the preference of cancer cells for glycolysis rather than oxidative phosphorylations (OXPHOs) when oxygen is not limited has been demonstrated by Otto Warburg [12], the aberrant glucose metabolism became one of the hallmarks of cancer. However there has been a paradigm shift towards so called reversed Warburg effect, since research showed that each cancer has its unique metabolic features, and some may synthesize ATP by means of OXPHOs [13]. A recent evidence suggests that CRC cells rely on the reversed Warburg effect [14, 15], which opened new perspectives for the identification of new molecular therapeutic targets, among them FA oxidation [34] and cardiovascular diseases [35]. A growing number of studies analyzed the relation between lipids and various malignancies: breast cancer [36, 37], prostate cancer [38, 39], ovarian cancer [177]. The authors of one lipidomic study demonstrated considerable alterations of several complex plasma lipids in CRC patients, and based on the analysis of receiver operating characteristic (ROC) curve proposed phosphatidylglycerol PG-18:0/16:0, sphingomyelin SM-d18:1/24:1 (42:2), ceramide Cer-d18:1/26:4 (elevated), LPC-18:3, LPC-18:2, phosphorylethanolamines PE-18:2/18:1, PE-18:1/20:2 and SM-38:8 (decreased) as biomarkers of this malignancy [89]. The use of biomarker clusters may have greater discriminative power than single molecules. In one study, patients with early CRC were identified accurately based on their serum levels of palmitic amide, oleamide, hexadecanedioic acid, 12-hydroxystearic acid, 20:3 n-3, 14:0, lysophosphatidic acid LPA-16:0, LPA-18:0 and LPC-16:0, with the area under the ROC curve equal 0.991, 0.981 sensitivity and 1.000 specificity [178]. Similar approach, with a panel of various metabolites, among them lipids, was also used to predict the recurrence and spread of CRC and survival in patients with this malignancy [206]. In another study, rectal cancer patients showed a significant increase in serum TG 56:6, 52:2 and 52:1, but it must be stressed that the study group was relatively small [89]. The authors of most studies analyzing TG levels in blood and tissues of CRC patients reported their overall concentrations but did not provide a detailed information about the content of specific FAs.
Specific fatty acids changes in adipose tissue of CRC patients
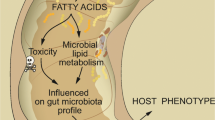
Although available data on FAs esterified in TGs are generally limited, some studies provided an insight into this lipid group, based on the analysis of adipose tissue. The latter is the main reservoir of TGs, capable of releasing them into bloodstream, and thus, it may influence the lipid profiles of various tissues. Many studies documented a relationship between obesity and colorectal cancer risk [58, 59]. Abdominal fat deposits, which can be expressed as the waist-to-hip ratio, seem to be a predominant “measure” of colorectal adenoma risk in men and women [207]. Moreover, as outlined recently in the review articles published by Himbert [208] and Masoodi [209]; also multifaceted interactions between adipose microenvironment and tumor, especially those mediated by proinflammatory factors, raise a growing interest of researchers. Thus, adipose tissue is no longer considered a merely energy reservoir, but also a source of various signaling molecules, adipokines [210], and FAs with proinflammatory properties that can modulate immune cells [211] or activating autophagy [212]. Furthermore, adipose tissue is no longer analyzed as a single entity, but as two distinct compartments, visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT). Furthermore, studies of SAT sometimes consider additional heterogenic nature of this tissue, with two distinct layers, deep and superficial one, that differ in terms of various parameters, e.g. the intensity of lipolysis [213,214,215,216]. Surprisingly, however, only few previous studies analyzed a link between CRC occurrence or progression and the content of some specific FAs in adipose tissue, showing some significant changes of their levels [92, 213, 217, 218].
The authors of one study published in 1988 found no significant intergroup differences in the content of seven major FAs determined by means of GLC-FID in SAT and RBCs from 49 CRC patients and 49 sex- and age-matched controls [218]. Also another case-control study conducted by Giuliani et al. [92] showed no significant differences in total SFAs or MUFAs content between SAT and VAT for both controls and patient. Total SFA content in VAT and total MUFA content in SAT turned out to be higher in CRC patients than in the controls (p < 0.001). Among specific PUFAs, CRC patients presented with higher levels of visceral 18:3 n-3 whereas lower 18:4 n-3 than the controls. Furthermore, the study showed that in CRC patients, the level of n-6 PUFA, 18:2 n-6, was significantly higher in SAT than in VAT. Finally, the total content of n-6 PUFAs (LA + GLA + DGLA + AA) in SAT was shown to be higher in healthy controls than in CRC patients.
A somehow different approach was presented by Cottet et al. [217], who analyzed subcutaneous adipose tissue FAs based on the estimated activity of the enzymes involved in their metabolism. Therein the putative marker for ELVOL5 + Δ-6-desaturase activities estimated by 20:3 n-6 to 18:2 n-6 ratio as well as ELVOL2/5 activity (ratio of 22:4 n-6 to 20:4 n-6 and 22:5 n-3 to 20:5 n-3) were positively associated with CRC risk. No such association with CRC risk was observed on the basis of 18:1 n-9 to 16:1 n-9 ratio.
One limitation of adipose tissue studies is the method of sample preparation, which has already been shown to influence FA concentration [219]. Furthermore, adipose tissue collection is an invasive procedure, and hence, is unlikely to be applicable to large-scale studies.
Conclusions
Despite a decrease in mortality, CRC still remains a serious public health burden [26]. A growing number of CRCs are diagnosed in patients younger than 50 years [220, 221]. The reason for this alarming tendency is yet to be elucidated, but it may be a consequence of greater exposure to environmental factors, lesser physical activity and unfavorable dietary changes. Analysis of lipid metabolism in cancer patients may provide a better insight into metabolic disturbances that contribute to carcinogenesis. The fact that cancer cells require lipids to proliferate [20], may open new therapeutic perspectives: perhaps some specific pathways involved in the synthesis and storage of fatty acids might be targeted to prevent cancer development [24]. Furthermore, some metabolites of fatty acids are important signaling molecules involved in the maintenance of proinflammatory and anti-inflammatory equilibrium. Probably these are proinflammatory factors which constitute a link between obesity and CRC [208]. Moreover, obesity is associated with lipidome changes [32] that may predispose to the development of some related conditions, among them cancer. Alterations of FAs, their metabolites and lipid species containing FA chains can be observed in tumor microenvironment as well (Table 1). Some of those alterations, such as accumulation of PC-16:0/16:1, may be considered as cancer biomarkers [176]. Lipid profile alterations, e.g. presence of cerotic acid [22] or a decrease in the concentration of hydroxylated, polyunsaturated ultra-long-chain fatty acids [169], can be also found in the sera of CRC patients, differentiating between early and advanced stages of this malignancy [178], or serving as a predictor of survival [179]. However, the development of clinically useful lipid biomarkers requires consistent research methodology, and previous studies were quite heterogenous in this matter. Another drawback of previous studies is limited sample size which may hinder generalization of their results onto the whole population of CRC patients. Nevertheless, understanding of lipid alterations associated with CRC may define new directions in the diagnosis and treatment of this malignancy.
Abbreviations
- AA:
-
Arachidonic acid
- ABCA1:
-
ATP-binding cassette sub-family A 1
- ACSL1:
-
Long chain acyl-CoA synthetase 1
- AGPAT1:
-
1-acyl-sn-glycerol-3-phosphate acyltransferase alpha
- ALA:
-
α-linolenic acid
- AMPK:
-
5’AMP-activated protein kinase
- APC:
-
Adenomatous polyposis coli
- ATGL:
-
Adipose triacylglycerol lipase
- Cer:
-
Ceramide
- CerS:
-
Ceramidase synthase
- CHα:
-
Choline kinase α
- CLA:
-
Conjugated linoleic acid
- CoA:
-
Coenzyme A
- COX:
-
Cyclooxygenase
- cPLA2 :
-
Cytosolic phospholipase A2
- CRC:
-
Colorectal cancer
- CSC:
-
Cancer stem cell
- CYP450:
-
Cytochrome p450
- DGLA:
-
Dihomo-γ-linolenic acid
- DHA:
-
Docosahexaenoic acid
- EGFR:
-
Epidermal growth factor receptor
- ELOVL:
-
Fatty acid elongase
- EMT:
-
Epithelial-mesenchymal transition
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- FASN:
-
Fatty acid synthase
- FFA:
-
Free fatty acid
- FIT:
-
Fecal immunochemical test
- gFOBT:
-
Guaiac-based fecal occult blood test
- GLA:
-
γ-linolenic acid
- Glu:
-
Glucose
- HDL:
-
High density lipoprotein
- HETE:
-
Hydroxyeicosatetraenoic acid
- HNE:
-
4-hydroxynonenal
- hPULCFA:
-
Hydroxylated, polyunsaturated ultra-long-chain fatty acid
- isoP:
-
Isoprostane
- IκBα:
-
Kappa-light-chain-enhancer of activated B cells inhibitor
- JNK1:
-
c-Jun N-terminal protein kinase 1
- LA:
-
Linoleic acid
- Lac:
-
Lactose
- LCFA:
-
Long-chain fatty acid
- LDL:
-
Low density lipoprotein
- LOX:
-
Lipoxygenase
- LPA:
-
Lysophosphatidic acid
- LPC:
-
Lysophosphatidylcholine
- LPP:
-
Lipid peroxidation product
- LT:
-
Leukotriene
- MCFA:
-
Medium-chain fatty acid
- MDA:
-
Malondialdehyde
- MLC2:
-
Myosin regulatory light chain 2
- mTOR:
-
Mammalian target of rapamycin
- MUFA:
-
Monounsaturated fatty acid
- NEFA:
-
Non-esterified fatty acid
- NF-κB:
-
Kappa-light-chain-enhancer of activated B cells
- OA:
-
Oleic acid
- OXPHO:
-
Oxidative phosphorylation
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphorylethanolamine
- PG:
-
Prostaglandin
- PHL:
-
Phospholipid
- PKR:
-
Protein kinase R
- PL:
-
Polar lipid
- PPAR:
-
Peroxisome proliferator-activated receptor
- PUFA:
-
Polyunsaturated fatty acid
- RBC:
-
Red blood cell
- REIMS:
-
Rapid evaporative ionization mass spectrometry
- Rho/ROCK:
-
Rho/Rho-associated coiled-coil containing protein kinase
- ROC:
-
Receiver operating characteristic
- ROS:
-
Reactive oxygen species
- S1P:
-
Sphingosine-1-phosphate
- SAT:
-
Subcutaneous adipose tissue
- SCD:
-
Stearoyl-CoA desaturase
- SCFA:
-
Short-chain fatty acid
- SFA:
-
Saturated fatty acid
- SM:
-
Sphingomyelin
- SphK:
-
Sphingosine kinase
- SPL:
-
Sphingolipid
- SPM:
-
Specialized pro-resolving mediator
- TC:
-
Total cholesterol
- TG:
-
Triacylglycerol
- TLR:
-
Toll-like receptor
- VAT:
-
Visceral adipose tissue
- VLCFA:
-
Very long-chain fatty acid
- VLDL:
-
Very-low-density-lipoprotein
- YAMC:
-
Young adult mouse colonic
References
Stephenson DJ, Hoeferlin LA, Chalfant CE. Lipidomics in translational research and the clinical significance of lipid-based biomarkers. Transl Res. 2017;189:13–29. https://doi.org/10.1016/j.trsl.2017.06.006.
Hamilton SR, Rubio CA, Volgenstein B, Sobin LH, Kudo S, Fogt F, et al. Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen LA, editors. World health organization classification of Tumours. Pathology and genetics of Tumours of the digestive system. Lyon: IARC Press; 2000. p. 105–20.
Strum WB. Colorectal Adenomas. N Engl J Med. 2016;374:1065–75. https://doi.org/10.1056/NEJMra1513581.
Yamagishi H, Kuroda H, Imai Y, Hiraishi H. Molecular pathogenesis of sporadic colorectal cancers. Chin J Cancer. 2016;35:4. https://doi.org/10.1186/s40880-015-0066-y.
Grady WM. Genetic testing for high-risk colon cancer patients. Gastroenterology. 2003;124:1574–94. https://doi.org/10.1016/S0016-5085(03)00376-7.
Cappell MS. Pathophysiology, clinical presentation, and Management of Colon Cancer. Gastroenterol Clin N Am. 2008;37:1–24. https://doi.org/10.1016/j.gtc.2007.12.002.
Robbins DH, Itzkowitz SH. The molecular and genetic basis of colon cancer. Med Clin North Am. 2002;86:1467–95. https://doi.org/10.1016/S0025-7125(02)00084-6. Accessed 22 Feb 2018.
Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol. 2012;18:5171–80. https://doi.org/10.3748/wjg.v18.i37.5171.
Hanahan D, Weinberg RA. Hallmarks of Cancer: the next generation. Cell. 2011;144:646–74. https://doi.org/10.1016/J.CELL.2011.02.013.
Liu X, Jakubowski M, Hunt JL. KRAS gene mutation in colorectal Cancer is correlated with increased proliferation and spontaneous apoptosis. Am J Clin Pathol. 2011;135:245–52. https://doi.org/10.1309/AJCP7FO2VAXIVSTP.
Georgescu CV, Săftoiu A, Georgescu CC, Ciurea R, Ciurea T. Correlations of proliferation markers, p53 expression and histological findings in colorectal carcinoma. J Gastrointestin Liver Dis. 2007;16:133–9 http://www.ncbi.nlm.nih.gov/pubmed/17592558. Accessed 4 Jun 2018.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30. https://doi.org/10.1085/jgp.8.6.519. Accessed 23 Feb 2018.
Xu XD, Shao SX, Jiang HP, Cao YW, Wang YH, Yang XC, et al. Warburg effect or reverse Warburg effect? A review of Cancer metabolism. Oncol Res Treat. 2015;38:117–22. https://doi.org/10.1159/000375435.
Chekulayev V, Mado K, Shevchuk I, Koit A, Kaldma A, Klepinin A, et al. Metabolic remodeling in human colorectal cancer and surrounding tissues: alterations in regulation of mitochondrial respiration and metabolic fluxes. Biochem Biophys Reports. 2015;4:111–25. https://doi.org/10.1016/J.BBREP.2015.08.020.
Satoh K, Yachida S, Sugimoto M, Oshima M, Nakagawa T, Akamoto S, et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc Natl Acad Sci U S A. 2017;114:E7697–706. https://doi.org/10.1073/pnas.1710366114.
Fu Y, Liu S, Yin S, Niu W, **ong W, Tan M, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8:57813–25. https://doi.org/10.18632/oncotarget.18175.
Hao Y, Samuels Y, Li Q, Krokowski D, Guan B-J, Wang C, et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun. 2016;7:11971. https://doi.org/10.1038/ncomms11971.
Miyo M, Konno M, Nishida N, Sueda T, Noguchi K, Matsui H, et al. Metabolic adaptation to nutritional stress in human colorectal Cancer. Sci Rep. 2016;6:38415. https://doi.org/10.1038/srep38415.
Huang C, Freter C. Lipid metabolism, apoptosis and Cancer therapy. Int J Mol Sci. 2015;16:924–49. https://doi.org/10.3390/ijms16010924.
Zaytseva YY, Harris JW, Mitov MI, Kim JT, Butterfield DA, Lee EY, et al. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget. 2015;6:18891–904. https://doi.org/10.18632/oncotarget.3783.
Sánchez-Martínez R, Cruz-Gil S, García-Álvarez MS, Reglero G, Ramírez de Molina A. Complementary ACSL isoforms contribute to a non-Warburg advantageous energetic status characterizing invasive colon cancer cells. Sci Rep. 2017;7:11143. https://doi.org/10.1038/s41598-017-11612-3.
Mika A, Kobiela J, Czumaj A, Chmielewski M, Stepnowski P, Sledzinski T. Hyper-elongation in colorectal Cancer tissue - cerotic acid is a potential novel serum metabolic marker of colorectal malignancies. Cell Physiol Biochem. 2017;41:722–30.
Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogene. 2016;5:e189. https://doi.org/10.1038/oncsis.2015.49.
Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and Cancer. Cell Metab. 2013;18:153–61. https://doi.org/10.1016/J.CMET.2013.05.017.
Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76. https://doi.org/10.1186/s12943-017-0646-3.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. https://doi.org/10.3322/caac.21395.
Thélin C, Sikka S. Epidemiology of colorectal Cancer — incidence, lifetime risk factors statistics and temporal trends. In: Ettarh R, editor. Screening for colorectal Cancer with colonoscopy. London: IntechOpen Limited; 2015. p 61-77. https://www.intechopen.com/books/screening-for-colorectal-cancer-with-colonoscopy/epidemiology-of-colorectal-cancer-incidence-lifetime-risk-factors-statistics-and-temporal-trends
Kahi CJ, Rex DK. Current and future trends in colorectal cancer screening. Cancer Metastasis Rev. 2004;23:137–44. https://doi.org/10.1023/A:1025871231346. Accessed 21 Jan 2018.
Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut. 2015;64:1327–37. https://doi.org/10.1136/gutjnl-2014-308074.
Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJY, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–49. https://doi.org/10.1136/gutjnl-2014-309086.
Meikle PJ, Christopher MJ. Lipidomics is providing new insight into the metabolic syndrome and its sequelae. Curr Opin Lipidol. 2011;22:210–5. https://doi.org/10.1097/MOL.0b013e3283453dbe.
Mika A, Śledzinski T. Alterations of specific lipid groups in serum of obese humans: a review. Obes Rev. 2017;18:247–72. https://doi.org/10.1111/obr.12475.
Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90. https://doi.org/10.1002/hep.21763.
Markgraf DF, Al-Hasani H, Lehr S. Lipidomics-resha** the analysis and perception of type 2 diabetes. Int J Mol Sci. 2016;17:1841. https://doi.org/10.3390/ijms17111841.
Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129:1821–31. https://doi.org/10.1161/CIRCULATIONAHA.113.002500.
Yamashita Y, Nishiumi S, Kono S, Takao S, Azuma T, Yoshida M. Differences in elongation of very long chain fatty acids and fatty acid metabolism between triple-negative and hormone receptor-positive breast cancer. BMC Cancer. 2017;17:589. https://doi.org/10.1186/s12885-017-3554-4.
Yang B, Ren X-L, Fu Y-Q, Gao J-L, Li D. Ratio of n-3/n-6 PUFAs and risk of breast cancer: a meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer. 2014;14:105. https://doi.org/10.1186/1471-2407-14-105.
Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta - Mol Cell Biol Lipids. 2013;1831:1302–9. https://doi.org/10.1016/J.BBALIP.2013.04.011.
Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–32. https://doi.org/10.1016/j.ejca.2016.10.011.
Li J, Condello S, Thomes-Pepin J, Ma X, **a Y, Hurley TD, et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian Cancer stem cells. Cell Stem Cell. 2017;20:303–314.e5. https://doi.org/10.1016/j.stem.2016.11.004.
Zhang Y, Liu Y, Li L, Wei J, **ong S, Zhao Z. High resolution mass spectrometry coupled with multivariate data analysis revealing plasma lipidomic alteration in ovarian cancer in Asian women. Talanta. 2016;150:88–96. https://doi.org/10.1016/j.talanta.2015.12.021.
Li Z, Guan M, Lin Y, Cui X, Zhang Y, Zhao Z, et al. Aberrant lipid metabolism in hepatocellular carcinoma revealed by liver Lipidomics. Int J Mol Sci. 2017;18:2550. https://doi.org/10.3390/ijms18122550.
**a S-H, Wang J, Kang JX. Decreased n −6/ n −3 fatty acid ratio reduces the invasive potential of human lung cancer cells by downregulation of cell adhesion/invasion-related genes. Carcinogenesis. 2005;26:779–84. https://doi.org/10.1093/carcin/bgi019.
Swierczynski J, Hebanowska A, Sledzinski T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J Gastroenterol. 2014;20:2279. https://doi.org/10.3748/wjg.v20.i9.2279.
Piyarathna DWB, Rajendiran TM, Putluri V, Vantaku V, Soni T, von Rundstedt F-C, et al. Distinct Lipidomic landscapes associated with clinical stages of urothelial Cancer of the bladder. Eur Urol Focus. 2017. https://doi.org/10.1016/J.EUF.2017.04.005.
LIPID MAPS® Lipidomics Gateway http://www.lipidmaps.org/data/classification/LM_classification_exp.php. Accessed 26 Jun 2018.
Fahy E, Cotter D, Sud M, Subramaniam S. Lipid classification, structures and tools. Biochim Biophys Acta - Mol Cell Biol Lipids. 2011;1811:637–47. https://doi.org/10.1016/j.bbalip.2011.06.009.
Kimura T, Jennings W, Epand RM. Roles of specific lipid species in the cell and their molecular mechanism. Prog Lipid Res. 2016;62:75–92. https://doi.org/10.1016/J.PLIPRES.2016.02.001.
Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007;2:229–37. https://doi.org/10.2217/17460875.2.2.229.
Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57:943–54. https://doi.org/10.1194/jlr.R067629.
Jump DB. Mammalian fatty acid elongases. Methods Mol Biol. 2009;579:375–89. https://doi.org/10.1007/978-1-60761-322-0_19.
Tosi F, Sartori F, Guarini P, Olivieri O, Martinelli N. Delta-5 and Delta-6 desaturases: crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. In: Camps J, editor. Oxidative stress and inflammation in non-communicable diseases - molecular mechanisms and perspectives in therapeutics. Advances in experimental medicine and biology, vol. 824. Cham: Springer; 2014. p. 61–81. https://doi.org/10.1007/978-3-319-07320-0_7.
Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PRC. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 2003;38:391–8. https://doi.org/10.1007/s11745-003-1074-0.
Lands B, Bibus D, Stark KD. Dynamic interactions of n-3 and n-6 fatty acid nutrients. Prostaglandins, Leukot Essent Fat Acids. 2017. https://doi.org/10.1016/J.PLEFA.2017.01.012.
Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ. 2014;348:g2272. https://doi.org/10.1136/BMJ.G2272.
Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. https://doi.org/10.5402/2012/137289.
Ni Y, Zhao L, Yu H, Ma X, Bao Y, Rajani C, et al. Circulating unsaturated fatty acids delineate the metabolic status of obese individuals. EBioMedicine. 2015;2:1513–22. https://doi.org/10.1016/j.ebiom.2015.09.004.
Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933–47. https://doi.org/10.1136/gutjnl-2013-304701.
Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8:e53916. https://doi.org/10.1371/journal.pone.0053916.
Qin S, Yin J, Huang K. Free fatty acids increase intracellular lipid accumulation and oxidative stress by modulating PPARα and SREBP-1c in L-02 cells. Lipids. 2016;51:797–805. https://doi.org/10.1007/s11745-016-4160-y.
Lupachyk S, Watcho P, Hasanova N, Julius U, Obrosova IG. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: role for oxidative–nitrosative stress. Free Radic Biol Med. 2012;52:1255–63. https://doi.org/10.1016/j.freeradbiomed.2012.01.029.
Daniëls VW, Smans K, Royaux I, Chypre M, Swinnen JV, Zaidi N. Cancer cells differentially activate and thrive on de novo lipid synthesis pathways in a low-lipid environment. PLoS One. 2014;9:e106913. https://doi.org/10.1371/journal.pone.0106913.
Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M. Fatty acid synthase over expression is an Indicator of tumor aggressiveness and poor prognosis in renal cell carcinoma. J Urol. 2008;180:1137–40. https://doi.org/10.1016/j.juro.2008.04.135.
Stoiber K, Nagło O, Pernpeintner C, Zhang S, Koeberle A, Ulrich M, et al. Targeting de novo lipogenesis as a novel approach in anti-cancer therapy. Br J Cancer. 2018;118:43–51. https://doi.org/10.1038/bjc.2017.374.
Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–26. https://doi.org/10.1158/0008-5472.CAN-09-3871.
Chang L, Wu P, Senthilkumar R, Tian X, Liu H, Shen X, et al. Loss of fatty acid synthase suppresses the malignant phenotype of colorectal cancer cells by down-regulating energy metabolism and mTOR signaling pathway. J Cancer Res Clin Oncol. 2016;142:59–72. https://doi.org/10.1007/s00432-015-2000-8.
Francipane MG, Lagasse E. mTOR pathway in colorectal cancer: an update. Oncotarget. 2014;5:49–66. https://doi.org/10.18632/oncotarget.1548.
Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56. https://doi.org/10.1158/0008-5472.CAN-10-4058.
Wang H, ** Q, Wu G. Fatty acid synthase regulates invasion and metastasis of colorectal cancer via Wnt signaling pathway. Cancer Med. 2016;5:1599–606. https://doi.org/10.1002/cam4.711.
Cao D, Song X, Che L, Li X, Pilo MG, Vidili G, et al. Both de novo synthetized and exogenous fatty acids support the growth of hepatocellular carcinoma cells. Liver Int. 2017;37:80–9. https://doi.org/10.1111/liv.13183.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. https://doi.org/10.1136/gutjnl-2015-310912.
van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, et al. A saturated fatty acid–rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009;90:1656–64. https://doi.org/10.3945/ajcn.2009.27792.
Phillips CM, Kesse-Guyot E, McManus R, Hercberg S, Lairon D, Planells R, et al. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity–associated gene in adults. J Nutr. 2012;142:824–31. https://doi.org/10.3945/jn.111.153460.
Kennedy A, Martinez K, Chuang C-C, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4. https://doi.org/10.3945/jn.108.098269.
Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol. 2010;222:187–94. https://doi.org/10.1002/jcp.21936.
Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–13. https://doi.org/10.1194/jlr.D029546.
Li T-T, Ogino S, Qian ZR. Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy. World J Gastroenterol. 2014;20:17699–708. https://doi.org/10.3748/wjg.v20.i47.17699.
Mensink RP. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva; 2016. http://apps.who.int/iris/bitstream/10665/246104/1/9789241565349-eng.pdf. Accessed 17 Jan 2018
van Duijnhoven FJB, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EHJM, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European prospective investigation into Cancer and nutrition. Gut. 2011;60:1094–102. https://doi.org/10.1136/gut.2010.225011.
Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Rafaniello C, et al. Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta-analysis. Endocrine. 2013;44:634–47. https://doi.org/10.1007/s12020-013-9939-5.
Passarelli MN, Newcomb PA. Blood lipid concentrations and colorectal adenomas: a systematic review and meta-analysis of colonoscopy studies in Asia, 2000–2014. Am J Epidemiol. 2016;183:691–700. https://doi.org/10.1093/aje/kwv294.
Yao X, Tian Z. Dyslipidemia and colorectal cancer risk: a meta-analysis of prospective studies. Cancer Causes Control. 2015;26:257–68. https://doi.org/10.1007/s10552-014-0507-y.
Sidahmed E, Sen A, Ren J, Patel A, Turgeon DK, Ruffin MT, et al. Colonic saturated fatty acid concentrations and expression of COX-1, but not diet, predict prostaglandin E2 in Normal human Colon tissue. Nutr Cancer. 2016;68:1192–201. https://doi.org/10.1080/01635581.2016.1213866.
Hodge AM, Williamson EJ, Bassett JK, MacInnis RJ, Giles GG, English DR. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int J Cancer. 2015;137:1224–34. https://doi.org/10.1002/ijc.29479.
Kondo Y, Nishiumi S, Shinohara M, Hatano N, Ikeda A, Yoshie T, et al. Serum fatty acid profiling of colorectal cancer by gas chromatography/mass spectrometry. Biomark Med. 2011;5:451–60. https://doi.org/10.2217/bmm.11.41.
Zhang J, Zhang L, Ye X, Chen L, Zhang L, Gao Y, et al. Characteristics of fatty acid distribution is associated with colorectal cancer prognosis. Prostaglandins Leukot Essent Fat Acids. 2013;88:355–60. https://doi.org/10.1016/J.PLEFA.2013.02.005.
Butler LM, Yuan J-M, Huang JY, Su J, Wang R, Koh W-P, et al. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese health study. npj Precis Oncol. 2017;1:38. https://doi.org/10.1038/s41698-017-0040-z.
May-Wilson S, Sud A, Law PJ, Palin K, Tuupanen S, Gylfe A, et al. Pro-inflammatory fatty acid profile and colorectal cancer risk: a Mendelian randomisation analysis. Eur J Cancer. 2017;84:228–38. https://doi.org/10.1016/j.ejca.2017.07.034.
Shen S, Yang L, Li L, Bai Y, Cai C, Liu H. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J Chromatogr B. 2017;1068–1069:41–8. https://doi.org/10.1016/j.jchromb.2017.10.004.
Tamura K, Makino A, Hullin-Matsuda F, Kobayashi T, Furihata M, Chung S, et al. Novel Lipogenic enzyme ELOVL7 is involved in prostate Cancer growth through saturated Long-chain fatty acid metabolism. Cancer Res. 2009;69:8133–40. https://doi.org/10.1158/0008-5472.CAN-09-0775.
González-Bengtsson A, Asadi A, Gao H, Dahlman-Wright K, Jacobsson A. Estrogen enhances the expression of the polyunsaturated fatty acid Elongase Elovl2 via ERα in breast Cancer cells. PLoS One. 2016;11:e0164241. https://doi.org/10.1371/journal.pone.0164241.
Giuliani A, Ferrara F, Scimò M, Angelico F, Olivieri L, Basso L. Adipose tissue fatty acid composition and colon cancer: a case–control study. Eur J Nutr. 2014;53:1029–37. https://doi.org/10.1007/s00394-013-0605-8.
Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13:154. https://doi.org/10.1186/1476-511X-13-154.
Verberne L, Bach-Faig A, Buckland G, Serra-Majem L. Association between the Mediterranean diet and Cancer risk: a review of observational studies. Nutr Cancer. 2010;62:860–70. https://doi.org/10.1080/01635581.2010.509834.
Niso-Santano M, Malik SA, Pietrocola F, Bravo-San Pedro JM, Marino G, Cianfanelli V, et al. Unsaturated fatty acids induce non-canonical autophagy. EMBO J. 2015;34:1025–41. https://doi.org/10.15252/embj.201489363.
Burada F, Nicoli ER, Ciurea ME, Uscatu DC, Ioana M, Gheonea DI. Autophagy in colorectal cancer: an important switch from physiology to pathology. World J Gastrointest Oncol. 2015;7:271–84. https://doi.org/10.4251/wjgo.v7.i11.271.
Berger E, Nassra M, Atgié C, Plaisancié P, Géloën A. Oleic acid uptake reveals the rescued enterocyte phenotype of Colon Cancer Caco-2 by HT29-MTX cells in co-culture mode. Int J Mol Sci. 2017;18:1573. https://doi.org/10.3390/ijms18071573.
Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, et al. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem Biophys Res Commun. 2005;332:735–42. https://doi.org/10.1016/J.BBRC.2005.05.013.
Kim S-J, Choi H, Park S-S, Chang C, Kim E. Stearoyl CoA desaturase (SCD) facilitates proliferation of prostate cancer cells through enhancement of androgen receptor transactivation. Mol Cells. 2011;31:371–7. https://doi.org/10.1007/s10059-011-0043-5.
Kumar-Sinha C, Ignatoski KW, Lippman ME, Ethier SP, Chinnaiyan AM. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 2003;63:132–9 http://www.ncbi.nlm.nih.gov/pubmed/12517789. Accessed 21 Jan 2018.
Presler M, Wojtczyk-Miaskowska A, Schlichtholz B, Kaluzny A, Matuszewski M, Mika A, et al. Increased expression of the gene encoding stearoyl-CoA desaturase 1 in human bladder cancer. Mol Cell Biochem. 2018:1–8. https://doi.org/10.1007/s11010-018-3306-z.
Yahagi N, Shimano H, Hasegawa K, Ohashi K, Matsuzaka T, Najima Y, et al. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41:1316–22. https://doi.org/10.1016/j.ejca.2004.12.037.
Vargas T, Moreno-Rubio J, Herranz J, Cejas P, Molina S, González-Vallinas M, et al. ColoLipidGene: signature of lipid metabolism-related genes to predict prognosis in stage-II colon cancer patients. Oncotarget. 2015;6:7348–63. https://doi.org/10.18632/oncotarget.3130.
Byberg L, Kilander L, Warensjo Lemming E, Michaelsson K, Vessby B. Cancer death is related to high palmitoleic acid in serum and to polymorphisms in the SCD-1 gene in healthy Swedish men. Am J Clin Nutr. 2014;99:551–8. https://doi.org/10.3945/ajcn.113.065714.
Li J, Ren S, Piao H, Wang F, Yin P, Xu C, et al. Integration of lipidomics and transcriptomics unravels aberrant lipid metabolism and defines cholesteryl oleate as potential biomarker of prostate cancer. Sci Rep. 2016;6:20984. https://doi.org/10.1038/srep20984.
Pisanu ME, Noto A, De Vitis C, Morrone S, Scognamiglio G, Botti G, et al. Blockade of Stearoyl-CoA-desaturase 1 activity reverts resistance to cisplatin in lung cancer stem cells. Cancer Lett. 2017;406:93–104. https://doi.org/10.1016/j.canlet.2017.07.027.
Cruz-Gil S, Sanchez-Martinez R, Gomez de Cedron M, Martin-Hernandez R, Vargas T, Molina S, et al. Targeting the lipid metabolic axis ACSL/SCD in colorectal cancer progression by therapeutic miRNAs: miR-19b-1 role. J Lipid Res. 2018;59:14–24. https://doi.org/10.1194/jlr.M076752.
Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20:9872–81. https://doi.org/10.3748/wjg.v20.i29.9872.
Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937–47. https://doi.org/10.3748/WJG.14.3937.
Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–9S. https://doi.org/10.3945/jn.111.155259.
Hassanzadeh P. Colorectal cancer and NF-κB signaling pathway. Gastroenterol Hepatol from Bed to Bench. 2011;4:127–32 http://www.ncbi.nlm.nih.gov/pubmed/24834170. Accessed 21 Dec 2017.
Mullen A, Loscher CE, Roche HM. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J Nutr Biochem. 2010;21:444–50. https://doi.org/10.1016/J.JNUTBIO.2009.02.008.
Hall MN, Chavarro JE, Lee I-M, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomark Prev. 2008;17:1136–43. https://doi.org/10.1158/1055-9965.EPI-07-2803.
Song M, Zhang X, Meyerhardt JA, Giovannucci EL, Ogino S, Fuchs CS, et al. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017;66:1790–6. https://doi.org/10.1136/gutjnl-2016-311990.
Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. 2012;107:S228–39. https://doi.org/10.1017/S0007114512001614.
Mocellin MC, Camargo CQ, Nunes EA, Fiates GMR, Trindade EBSM. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr. 2016;35:359–69. https://doi.org/10.1016/j.clnu.2015.04.013.
Hamilton K, Brooks P, Holmes M, Cunningham J, Russell FD. Evaluation of the composition of omega-3 fatty acids in dietary oil supplements. Nutr Diet. 2010;67:182–9. https://doi.org/10.1111/j.1747-0080.2010.01453.x.
Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–76S. https://doi.org/10.1093/ajcn/83.6.1467S.
Shaikh SR. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem. 2012;23:101–5. https://doi.org/10.1016/j.jnutbio.2011.07.001.
Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:43–7. https://doi.org/10.1016/j.plefa.2012.03.008.
Baillat G, Siret C, Delamarre E, Luis J. Early adhesion induces interaction of FAK and Fyn in lipid domains and activates raft-dependent Akt signaling in SW480 colon cancer cells. Biochim Biophys Acta - Mol Cell Res. 2008;1783:2323–31. https://doi.org/10.1016/J.BBAMCR.2008.08.008.
Staubach S, Hanisch F-G. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. 2011;8:263–77. https://doi.org/10.1586/epr.11.2.
Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLoS One. 2012;7:e39682. https://doi.org/10.1371/journal.pone.0039682.
Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–53. https://doi.org/10.1093/jn/137.3.548. Accessed 9 Jan 2018.
Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: current treatment paradigms, the importance of diet, and the role of chemoprevention. World J Clin Oncol. 2015;6:133–41. https://doi.org/10.5306/wjco.v6.i5.133.
Xu Y, Qi J, Yang X, Wu E, Qian SY. Free radical derivatives formed from cyclooxygenase-catalyzed dihomo-γ-linolenic acid peroxidation can attenuate colon cancer cell growth and enhance 5-fluorouracil’s cytotoxicity. Redox Biol. 2014;2:610–8. https://doi.org/10.1016/j.redox.2014.01.022.
Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. https://doi.org/10.1155/2012/539426.
Sakai M, Kakutani S, Horikawa C, Tokuda H, Kawashima H, Shibata H, et al. Arachidonic acid and cancer risk: a systematic review of observational studies. BMC Cancer. 2012;12:606. https://doi.org/10.1186/1471-2407-12-606.
Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, et al. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res. 2011;31:1–8. https://doi.org/10.1016/J.NUTRES.2011.01.002.
Yang K, Li H, Dong J, Dong Y, Wang C-Z. Expression profile of polyunsaturated fatty acids in colorectal cancer. World J Gastroenterol. 2015;21:2405–12. https://doi.org/10.3748/wjg.v21.i8.2405.
Marventano S, Kolacz P, Castellano S, Galvano F, Buscemi S, Mistretta A, et al. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? Int J Food Sci Nutr. 2015;66:611–22. https://doi.org/10.3109/09637486.2015.1077790.
Lee JM, Lee H, Kang S, Park WJ. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients. 2016;8:23. https://doi.org/10.3390/nu8010023.
Chen C, Nagana Gowda GA, Zhu J, Deng L, Gu H, Chiorean EG, et al. Altered metabolite levels and correlations in patients with colorectal cancer and polyps detected using seemingly unrelated regression analysis. Metabolomics. 2017;13:125. https://doi.org/10.1007/s11306-017-1265-0.
Zhu J, Djukovic D, Deng L, Gu H, Himmati F, Chiorean EG, et al. Colorectal Cancer detection using targeted serum metabolic profiling. J Proteome Res. 2014;13:4120–30. https://doi.org/10.1021/pr500494u.
Wang X, Lin H, Gu Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. https://doi.org/10.1186/1476-511X-11-25.
Rainer L, Heiss CJ. Conjugated linoleic acid: health implications and effects on body composition. J Am Diet Assoc. 2004;104:963–8. https://doi.org/10.1016/J.JADA.2004.03.016.
Evans NP, Misyak SA, Schmelz EM, Guri AJ, Hontecillas R, Bassaganya-Riera J. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARgamma. J Nutr. 2010;140:515–21. https://doi.org/10.3945/jn.109.115642.
Uma Maheswari Devi P, DH K, Uma Maheswari Devi P. Probiotic conjugated linoleic acid inhibits COX-2 inflammatory pathway. J Pharm Res. 2017;11:767–74 http://jprsolutions.info/files/final-file-59508c71f04fb3.23475784.pdf. Accessed 23 Jan 2018.
Yang B, Chen H, Stanton C, Ross RP, Zhang H, Chen YQ, et al. Review of the roles of conjugated linoleic acid in health and disease. J Funct Foods. 2015;15:314–25. https://doi.org/10.1016/J.JFF.2015.03.050.
Pierre A-S, Minville-Walz M, Fèvre C, Hichami A, Gresti J, Pichon L, et al. Trans-10, cis-12 conjugated linoleic acid induced cell death in human colon cancer cells through reactive oxygen species-mediated ER stress. Biochim Biophys Acta - Mol Cell Biol Lipids. 2013;1831:759–68. https://doi.org/10.1016/J.BBALIP.2013.01.005.
Faramarzi E, Mahdavi R, Mohammad-Zadeh M, Nasirimotlagh B, Sanaie S. Effect of conjugated linoleic acid supplementation on quality of life in rectal cancer patients undergoing preoperative Chemoradiotherapy. Pakistan J Med Sci. 2017;33:383–8. https://doi.org/10.12669/pjms.332.11925.
Mohammadzadeh M, Faramarzi E, Mahdavi R, Nasirimotlagh B, Asghari Jafarabadi M. Effect of conjugated linoleic acid supplementation on inflammatory factors and matrix metalloproteinase enzymes in rectal Cancer patients undergoing Chemoradiotherapy. Integr Cancer Ther. 2013;12:496–502. https://doi.org/10.1177/1534735413485417.
Rastmanesh R. An urgent need to include risk–benefit analysis in clinical trials investigating conjugated linoleic acid supplements in cancer patients. Contemp Clin Trials. 2011;32:69–73. https://doi.org/10.1016/j.cct.2010.09.005.
Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc Pharmacol. 2015;71:40–56. https://doi.org/10.1016/J.VPH.2015.03.005.
Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. https://doi.org/10.1002/jbt.10058.
Quiñonez-Flores CM, González-Chávez SA, Nájera DDR, Pacheco-Tena C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res Int. 2016;2016:6097417. https://doi.org/10.1155/2016/6097417.
Wang Z, Li Z, Ye Y, **e L, Li W. Oxidative stress and liver Cancer: etiology and therapeutic targets. Oxidative Med Cell Longev. 2016;2016:1–10. https://doi.org/10.1155/2016/7891574.
Jezierska-Drutel A, Rosenzweig SA, Neumann CA. Role of oxidative stress and the microenvironment in breast cancer development and progression. Adv Cancer Res. 2013;119:107–25. https://doi.org/10.1016/B978-0-12-407190-2.00003-4.
Oh B, Figtree G, Costa D, Eade T, Hruby G, Lim S, et al. Oxidative stress in prostate cancer patients: a systematic review of case control studies. Prostate Int. 2016;4:71–87. https://doi.org/10.1016/j.prnil.2016.05.002.
Carini F, Mazzola M, Rappa F, Jurjus A, Geagea AG, Al Kattar S, et al. Colorectal carcinogenesis: role of oxidative stress and antioxidants. Anticancer Res. 2017;37:4759–66. https://doi.org/10.21873/anticanres.11882.
Perše M. Oxidative stress in the pathogenesis of colorectal cancer: cause or consequence? Biomed Res Int. 2013;2013:725710. https://doi.org/10.1155/2013/725710.
Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800. https://doi.org/10.1096/fj.04-2330rev.
Zhang L, Chen B, Zhang J, Li J, Yang Q, Zhong Q, et al. Serum polyunsaturated fatty acid metabolites as useful tool for screening potential biomarker of colorectal cancer. Prostaglandins Leukot Essent Fat Acids. 2017;120:25–31. https://doi.org/10.1016/J.PLEFA.2017.04.003.
Skrzydlewska E, Sulkowski S, Koda M, Zalewski B, Kanczuga-Koda L, Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403–6. https://doi.org/10.3748/WJG.V11.I3.403.
Zhong H, Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–9. https://doi.org/10.1016/J.REDOX.2014.12.011.
Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426–33. https://doi.org/10.1074/jbc.M212549200.
Pizzimenti S, Menegatti E, Berardi D, Toaldo C, Pettazzoni P, Minelli R, et al. 4-Hydroxynonenal, a lipid peroxidation product of dietary polyunsaturated fatty acids, has anticarcinogenic properties in colon carcinoma cell lines through the inhibition of telomerase activity. J Nutr Biochem. 2010;21:818–26. https://doi.org/10.1016/j.jnutbio.2009.06.005.
Leslie CC. Cytosolic phospholipase A2: physiological function and role in disease. J Lipid Res. 2015;56:1386–402. https://doi.org/10.1194/jlr.R057588.
Dichlberger A, Schlager S, Maaninka K, Schneider WJ, Kovanen PT. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J Lipid Res. 2014;55:2471–8. https://doi.org/10.1194/jlr.M048553.
Ostermann AI, Schebb NH. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct. 2017;8:2355–67. https://doi.org/10.1039/C7FO00403F.
Buczynski MW, Dumlao DS, Dennis EA. Thematic review series: proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–38. https://doi.org/10.1194/jlr.R900004-JLR200.
Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of Oxylipins derived from dietary PUFAs. Adv Nutr. 2015;6:513–40. https://doi.org/10.3945/an.114.007732.
Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal Cancer stem cell expansion and metastasis in mice. Gastroenterology. 2015;149:1884–1895.e4. https://doi.org/10.1053/J.GASTRO.2015.07.064.
Stadler S, Nguyen CH, Schachner H, Milovanovic D, Holzner S, Brenner S, et al. Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca2+ signalling. Cell Mol Life Sci. 2017;74:1907–21. https://doi.org/10.1007/s00018-016-2441-5.
Savari S, Vinnakota K, Zhang Y, Sjölander A. Cysteinyl leukotrienes and their receptors: bridging inflammation and colorectal cancer. World J Gastroenterol. 2014;20:968–77. https://doi.org/10.3748/wjg.v20.i4.968.
Chen GG, Xu H, Lee JFY, Subramaniam M, Leung KL, Wang SH, et al. 15-hydroxy-eicosatetraenoic acid arrests growth of colorectal cancer cells via a peroxisome proliferator-activated receptor gamma-dependent pathway. Int J Cancer. 2003;107:837–43. https://doi.org/10.1002/ijc.11447.
Fedirko V, McKeown-Eyssen G, Serhan CN, Barry EL, Sandler RS, Figueiredo JC, et al. Plasma lipoxin A4 and resolvin D1 are not associated with reduced adenoma risk in a randomized trial of aspirin to prevent colon adenomas. Mol Carcinog. 2017;56:1977–83. https://doi.org/10.1002/mc.22629.
Ritchie SA, Tonita J, Alvi R, Lehotay D, Elshoni H, Myat S, et al. Low-serum GTA-446 anti-inflammatory fatty acid levels as a new risk factor for colon cancer. Int J Cancer. 2013;132:355–62. https://doi.org/10.1002/ijc.27673.
Ritchie SA, Heath D, Yamazaki Y, Grimmalt B, Kavianpour A, Krenitsky K, et al. Reduction of novel circulating long-chain fatty acids in colorectal cancer patients is independent of tumor burden and correlates with age. BMC Gastroenterol. 2010;10:140. https://doi.org/10.1186/1471-230X-10-140.
Hata T, Takemasa I, Takahashi H, Haraguchi N, Nishimura J, Hata T, et al. Downregulation of serum metabolite GTA-446 as a novel potential marker for early detection of colorectal cancer. Br J Cancer. 2017;117:227–32. https://doi.org/10.1038/bjc.2017.163.
Perttula K, Edmands WMB, Grigoryan H, Cai X, Iavarone AT, Gunter MJ, et al. Evaluating ultra-long-chain fatty acids as biomarkers of colorectal Cancer risk. Cancer Epidemiol Biomark Prev. 2016;25:1216–23. https://doi.org/10.1158/1055-9965.EPI-16-0204.
Okuno M, Hamazaki K, Ogura T, Kitade H, Matsuura T, Yoshida R, et al. Abnormalities in fatty acids in plasma, erythrocytes and adipose tissue in Japanese patients with colorectal cancer. In Vivo (Brooklyn). 2013;27:203–10 http://www.ncbi.nlm.nih.gov/pubmed/23422479. Accessed 27 Nov 2017.
Pickens CA, Lane-Elliot A, Comstock SS, Fenton JI. Altered saturated and monounsaturated plasma phospholipid fatty acid profiles in adult males with Colon adenomas. Cancer Epidemiol Biomark Prev. 2016;25:498–506. https://doi.org/10.1158/1055-9965.EPI-15-0696.
Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52:165–74. https://doi.org/10.1016/j.plipres.2012.10.004.
Guo S, Wang Y, Zhou D, Li Z. Significantly increased monounsaturated lipids relative to polyunsaturated lipids in six types of cancer microenvironment are observed by mass spectrometry imaging. Sci Rep. 2014;4:5959. https://doi.org/10.1038/srep05959.
Kurabe N, Hayasaka T, Ogawa M, Masaki N, Ide Y, Waki M, et al. Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci. 2013;104:1295–302. https://doi.org/10.1111/cas.12221.
Mirnezami R, Spagou K, Vorkas PA, Lewis MR, Kinross J, Want E, et al. Chemical map** of the colorectal cancer microenvironment via MALDI imaging mass spectrometry (MALDI-MSI) reveals novel cancer-associated field effects. Mol Oncol. 2014;8:39–49. https://doi.org/10.1016/J.MOLONC.2013.08.010.
Li F, Qin X, Chen H, Qiu L, Guo Y, Liu H, et al. Lipid profiling for early diagnosis and progression of colorectal cancer using direct-infusion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom. 2013;27:24–34. https://doi.org/10.1002/rcm.6420.
Qiu Y, Cai G, Zhou B, Li D, Zhao A, **e G, et al. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin Cancer Res. 2014;20:2136–46. https://doi.org/10.1158/1078-0432.CCR-13-1939.
Mansilla F, da Costa K-A, Wang S, Kruhøffer M, Lewin TM, Ørntoft TF, et al. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) overexpression in human colorectal cancer. J Mol Med. 2009;87:85–97. https://doi.org/10.1007/s00109-008-0409-0.
Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. https://doi.org/10.1007/978-1-4419-6741-1_1.
Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51:50–62. https://doi.org/10.1016/J.PLIPRES.2011.11.001.
Chen L, Chen H, Li Y, Li L, Qiu Y, Ren J. Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol Rep. 2015;34:447–54. https://doi.org/10.3892/or.2015.3973.
Chen L, Ren J, Yang L, Li Y, Fu J, Li Y, et al. Stearoyl-CoA desaturase-1 mediated cell apoptosis in colorectal cancer by promoting ceramide synthesis. Sci Rep. 2016;6:19665. https://doi.org/10.1038/srep19665.
Hartmann D, Lucks J, Fuchs S, Schiffmann S, Schreiber Y, Ferreirós N, et al. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int J Biochem Cell Biol. 2012;44:620–8. https://doi.org/10.1016/J.BIOCEL.2011.12.019.
Hartmann D, Wegner M-S, Wanger RA, Ferreirós N, Schreiber Y, Lucks J, et al. The equilibrium between long and very long chain ceramides is important for the fate of the cell and can be influenced by co-expression of CerS. Int J Biochem Cell Biol. 2013;45:1195–203. https://doi.org/10.1016/J.BIOCEL.2013.03.012.
Yabu T, Shiba H, Shibasaki Y, Nakanishi T, Imamura S, Touhata K, et al. Stress-induced ceramide generation and apoptosis via the phosphorylation and activation of nSMase1 by JNK signaling. Cell Death Differ. 2015;22:258–73. https://doi.org/10.1038/cdd.2014.128.
Woodcock J. Sphingosine and ceramide signalling in apoptosis. IMBMB Life. 2006;58:462–6. https://doi.org/10.1080/15216540600871118.
Hait NC, Maiti A. The role of Sphingosine-1-phosphate and Ceramide-1-phosphate in inflammation and Cancer. Mediat Inflamm. 2017;2017:4806541. https://doi.org/10.1155/2017/4806541.
Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2008;23:405–14. https://doi.org/10.1096/fj.08-117572.
Long J, **e Y, Yin J, Lu W, Fang S. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumor Biol. 2016;37:6831–6. https://doi.org/10.1007/s13277-015-4542-4.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. https://doi.org/10.1172/JCI39104.
Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–16. https://doi.org/10.1038/nrc1411.
Jelonek K, Ros M, Pietrowska M, Wildak P. Cancer biomarkers and mass spectrometry-based analyses of phospholipids in body fluids. Clin Lipidol. 2013;8:137–50. https://doi.org/10.2217/clp.12.79.
Bandu R, Mok HJ, Kim KP. Phospholipids as cancer biomarkers: mass spectrometry-based analysis. Mass Spectrom Rev. 2016. https://doi.org/10.1002/mas.21510.
Poss J, Custodis F, Werner C, Weingartner O, Bohm M, Laufs U. Cardiovascular disease and dyslipidemia: beyond LDL. Curr Pharm Des. 2011;17:861–70. https://doi.org/10.2174/138161211795428858.
Yang MH, Rampal S, Sung J, Choi Y-H, Son HJ, Lee JH, et al. The Association of Serum Lipids with Colorectal Adenomas. Am J Gastroenterol. 2013;108:833–41. https://doi.org/10.1038/ajg.2013.64.
Kim BC, Shin A, Hong CW, Sohn DK, Han KS, Ryu KH, et al. Association of colorectal adenoma with components of metabolic syndrome. Cancer Causes Control. 2012;23:727–35. https://doi.org/10.1007/s10552-012-9942-9.
Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjørge T, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (me-can) collaborative study. Cancer Causes Control. 2011;22:291–9. https://doi.org/10.1007/s10552-010-9697-0.
Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, et al. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009;18:240–7. https://doi.org/10.1097/cej.0b013e3283240460.
Agnoli C, Grioni S, Sieri S, Sacerdote C, Vineis P, Tumino R, et al. Colorectal cancer risk and dyslipidemia: a case–cohort study nested in an Italian multicentre cohort. Cancer Epidemiol. 2014;38:144–51. https://doi.org/10.1016/J.CANEP.2014.02.002.
Ulmer H, Borena W, Rapp K, Klenk J, Strasak A, Diem G, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer. 2009;101:1202–6. https://doi.org/10.1038/sj.bjc.6605264.
Coppola J-A, Shrubsole MJ, Cai Q, Smalley WE, Dai Q, Ness RM, et al. Plasma lipid levels and colorectal adenoma risk. Cancer Causes Control. 2015;26:635–43. https://doi.org/10.1007/s10552-015-0555-y.
Davis-Yadley AH, Lipka S, Shen H, Devanney V, Swarup S, Barnowsky A, et al. Ethnic disparities in the risk of colorectal adenomas associated with lipid levels: a retrospective multiethnic study. J Gastrointest Cancer. 2015;46:29–35. https://doi.org/10.1007/s12029-014-9671-2.
Ikeda K, Mutoh M, Teraoka N, Nakanishi H, Wakabayashi K, Taguchi R. Increase of oxidant-related triglycerides and phosphatidylcholines in serum and small intestinal mucosa during development of intestinal polyp formation in min mice. Cancer Sci. 2011;102:79–87. https://doi.org/10.1111/j.1349-7006.2010.01754.x.
Alexander J, Louise Gildea B, Julia Balog B, Abigail Speller B, James McKenzie B, Laura Muirhead B, et al. A novel methodology for in vivo endoscopic phenoty** of colorectal cancer based on real-time analysis of the mucosal lipidome: a prospective observational study of the iKnife. Surg Endosc. 2017;31:1361–70. https://doi.org/10.1007/s00464-016-5121-5.
Nam SY, Kim BC, Han KS, Ryu KH, Park BJ, Kim HB, et al. Abdominal visceral adipose tissue predicts risk of colorectal adenoma in both sexes. Clin Gastroenterol Hepatol. 2010;8:443–50. https://doi.org/10.1016/j.cgh.2010.02.001.
Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S, Ulrich CM. Signals from the adipose microenvironment and the obesity-Cancer link-a systematic review. Cancer Prev Res. 2017;10:494–506. https://doi.org/10.1158/1940-6207.CAPR-16-0322.
Masoodi M, Kuda O, Rossmeisl M, Flachs P, Kopecky J. Lipid signaling in adipose tissue: connecting inflammation & metabolism. Biochim Biophys Acta - Mol Cell Biol Lipids. 2015;1851:503–18. https://doi.org/10.1016/J.BBALIP.2014.09.023.
Jacobi D, Stanya KJ, Lee C-H. Adipose tissue signaling by nuclear receptors in metabolic complications of obesity. Adipocyte. 2012;1:4–12. https://doi.org/10.4161/adip.19036.
Del Cornò M, D’Archivio M, Conti L, Scazzocchio B, Varì R, Donninelli G, et al. Visceral fat adipocytes from obese and colorectal cancer subjects exhibit distinct secretory and ω6 polyunsaturated fatty acid profiles and deliver immunosuppressive signals to innate immunity cells. Oncotarget. 2016;7:63093–105. https://doi.org/10.18632/oncotarget.10998.
Wen Y-A, **ng X, Harris JW, Zaytseva YY, Mitov MI, Napier DL, et al. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8:e2593. https://doi.org/10.1038/cddis.2017.21.
Liesenfeld DB, Grapov D, Fahrmann JF, Salou M, Scherer D, Toth R, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. Am J Clin Nutr. 2015;102:433–43. https://doi.org/10.3945/ajcn.114.103804.
Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37:821–9. https://doi.org/10.2337/dc13-1353.
Monzon JR, Basile R, Heneghan S, Udupi V, Green A. Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obes Res. 2002;10:266–9. https://doi.org/10.1038/oby.2002.36.
Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot*. Obesity. 2007;15:1933–43. https://doi.org/10.1038/oby.2007.231.
Cottet V, Vaysse C, Scherrer M-L, Ortega-Deballon P, Lakkis Z, Delhorme J-B, et al. Fatty acid composition of adipose tissue and colorectal cancer: a case-control study. Am J Clin Nutr. 2015;101:192–201. https://doi.org/10.3945/ajcn.114.088948.
Neoptolemos JP, Clayton H, Heagerty AM, Nicholson MJ, Johnson B, Mason J, et al. Dietary fat in relation to fatty acid composition of red cells and adipose tissue in colorectal cancer. Br J Cancer. 1988;58:575–9. https://doi.org/10.1038/bjc.1988.262.
Ibáñez C, Simó C, Palazoglu M, Cifuentes A. GC-MS based metabolomics of colon cancer cells using different extraction solvents. Anal Chim Acta. 2017;986:48–56. https://doi.org/10.1016/j.aca.2017.07.019.
Inra JA, Syngal S. Colorectal Cancer in Young adults. Dig Dis Sci. 2015;60:722–33. https://doi.org/10.1007/s10620-014-3464-0.
Sheneman DW, Finch JL, Messersmith WA, Leong S, Goodman KA, Davis SL, et al. The impact of young adult colorectal cancer: incidence and trends in Colorado. Color Cancer. 2017;6:49–56. https://doi.org/10.2217/crc-2017-0008.
Acknowledgements
Not applicable.
Funding
This study was supported by the Poland National Science Centre grant no. 2016/22/E/NZ4/00665.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
AP and AM studied the literature and prepared the manuscript; AP, JK, PS, TS and AM wrote and reviewed the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
AP, AM and PS are chemists
JK is a surgeon
TS is a biochemist
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pakiet, A., Kobiela, J., Stepnowski, P. et al. Changes in lipids composition and metabolism in colorectal cancer: a review. Lipids Health Dis 18, 29 (2019). https://doi.org/10.1186/s12944-019-0977-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-019-0977-8