Abstract
Background
Cucumber downy mildew is among the most important diseases that can disrupt cucumber production. Propamocarb, also known as propyl-[3-(dimethylamino)propyl]carbamate (PM), is a systemic carbamate fungicide pesticide that is widely applied in agricultural production because of its high efficiency of pathogens control, especially cucumber downy mildew. However, residual PM can remain in cucumbers after the disease has been controlled. To explore the molecular mechanisms of PM retention, cucumber cultivars ‘D9320’ (with the highest residual PM content) and ‘D0351’ (lowest residual PM content) were studied. High-throughput tag-sequencing (Tag-Seq) results showed that the CsDIR16 gene was related to PM residue, which was verified using transgenic technology.
Results
We investigated the activity of a dirigent cucumber protein encoded by the CsDIR16 in gene response to stress induced by PM treatment. Gene-expression levels of CsDIR16 were up-regulated in the fruits, leaves, and stems of ‘D0351’ plants in response to PM treatment. However, in cultivar ‘D9320’, CsDIR16 levels were down-regulated in the leaves and stems after PM treatment, with no statistically significant differences observed in the fruits. Induction by jasmonic acid, abscisic acid, polyethylene glycol 4000, NaCl, and Corynespora cassiicola Wei (Cor) resulted in CsDIR16 up-regulation in ‘D0351’ and ‘D9320’. Expression after salicylic acid treatment was up-regulated in ‘D0351’, but was down-regulated in ‘D9320’. CsDIR16 overexpression lowered PM residues, and these were more rapidly reduced in CsDIR16(+) transgenic ‘D9320’ plants than in wild-type ‘D9320’ and CsDIR16(−) transgenic plants.
Conclusions
Analyses of the CsDIR16-expression patterns in the cucumber cultivars with the highest and lowest levels of PM residue, and transgenic validation indicated that CsDIR16 plays a positive role in reducing PM residues. The findings of this study help understand the regulatory mechanisms occurring in response to PM stress in cucumbers and in establishing the genetic basis for develo** low-pesticide residue cucumber cultivars.
Similar content being viewed by others
Background
Pesticides are among the most widely used chemicals in the world. With their application in modern agriculture, up to 80% of crop yield were protected from pest and weeds [34]. DIRs lack a catalytically active (oxidative) center and function only as guiding proteins [35]. The mechanism of action is thought to involve capture of free radicals produced by the oxidation of coniferyl alcohol, and the FiDIR protein catalyze 8–8′ coupling to produce (+)-pinoresinol, the AtDIR5 and AtDIR6 proteins catalyze 8–8′ coupling to produce (−)-pinoresinol [36,34].
Phylogenetic analysis and sequence alignment of CsDIR16. a Phylogenetic relationships of CsDIR16 with homologous proteins from other plant species. b Phylogenetic tree of all CsDIRs. The homologous sequences were from species showing the highest CsDIR16 sequence similarities. The amino sequences were subjected to phylogenetic analysis using the neighbor-joining method in MEGA7.0.20 software, with 1000 bootstrap replicates. The GenBank accession numbers are as follows: AtDIR2 (NP_199065.1), AtDIR7 (NP_187974.1), AtDIR9 (NP_181475.1), AtDIR10 (Q9SIA8.1), AtDIR13 (NP_192858.1), AtDIR16 (NP_189044.1), AtDIR18 (NP_193094.1), AtDIR19 (NP_176113.1), CmDIR7 (XP_008449790.1), CmDIR20 (XP_008449789.1), OsDIR02 (BAS72036.1), CsDIR1 (KGN63770.1), CsDIR2 (XP_004138340.1), CsDIR3 (KGN64478.1), CsDIR4 (XP_004147025.1), CsDIR5 (XP_004147026.1), CsDIR6 (XP_011649995.1), CsDIR7 (KGN55603.1), CsDIR8 (XP_004134021.1), CsDIR9 (XP_004134245.1), CsDIR10 (XP_004153955.1), CsDIR11 (XP_004146539.1), CsDIR12 (XP_004146540.1), CsDIR13 (XP_004146540.1), CsDIR14 (KGN54084.1), CsDIR15 (KGN54085.1), CsDIR16 (XP_004142157.2), CsDIR17 (XP_004142156.1), CsDIR18 (XP_011653564.1), CsDIR19 (KGN50509.1), CsDIR20 (XP_004147710.2), CsDIR21 (XP_004140684.2), CsDIR22 (XP_004144592.1), CsDIR23 (XP_004139895.1)
Twenty-three transcripts were identified in the cucumber genome sequence as possible members of the dirigent family and were named CsDIR1 to CsDIR23, based on their order in the cucumber genomic sequence (Additional file 1: Table S2). A phylogenetic tree of CsDIRs was constructed using Mega7.0 software (Fig. 3b). This analysis indicated that these proteins belong to 4 subfamilies (DIR-a, DIR-b, DIR-d, and DIR-e). Transcriptome analysis [23] showed that only 4 (CsDIR5, CsDIR7, CsDIR10, CsDIR16) of the 23 CsDIR genes in cucumber responded to PM treatment (Additional file 1: Table S3). Of these 4 genes, CsDIR16 had the highest expression level.
Subcellular localization of the CsDIR16 protein
The subcellular localization of the CsDIR16 protein was investigated using a CsDIR16-eGFP fusion gene driven by a 35S promoter; transient expression in Arabidopsis protoplast cells showed that the CsDIR16-eGFP fusion protein was enriched in the nucleus (Fig. 4). The results clearly indicated that CsDIR16 is a nuclear-localized protein.
Subcellular localization of the CsDIR16-pGII-EGFP fusion protein in Arabidopsis protoplasts. Images show protoplasts prepared from 3- to 4-week-old Arabidopsis leaves expressing CsDIR16-pGII-EGFP (upper row) or pGII-EGFP (bottom row). Bright-field illumination, GFP fluorescence, chlorophyll fluorescence, and an overlay of GFP and chlorophyll fluorescence are shown. Scale bars, 10 μm
Expression pattern of CsDIR16 in response to PM treatment
The expression patterns of CsDIR16 in the fruit of cultivars ‘D0351’ and ‘D9320’ were determined in control and PM-treated plants (Fig. 5a). In the ‘D0351’ cultivar, the expression level of CsDIR16 gene significantly increased at 3 h post-PM treatment, plateaued at 6 h, and then gradually decreased over time. The relative fold-changes of expression were 2.13, 1.97, 2.66, 2.43, and 13.77-fold at 0.5, 1, 3, 6, and 9 h after treatment, respectively. In contrast, CsDIR16 expression followed a different pattern in the ‘D9320’ cultivar. Peak CsDIR16 expression was seen at 3 h post-treatment, and no statistically significant differences were observed compared to control plants except at 9 h. Differences in CsDIR16 gene expression in response to PM were observed between the lowest PM-residue cultivar ‘D0351’ and the highest PM-residue cultivar ‘D9320’, indicating that the CsDIR16 gene might be closely associated with PM metabolism. CsDIR16 expression was induced by PM treatment in the fruit of ‘D0351’, suggesting that this gene serves a crucial role in metabolizing PM. However, no significant up-regulation of CsDIR16 was found in ‘D9320’, indicating that CsDIR16 is not the main metabolically related gene for PM residues in this cultivar.
Expression pattern of CsDIR16 in cucumber ‘D0351’ and ‘D9320’ after PM treatment and different stresses. a Relative transcript levels in fruits at 0.5, 1, 3, 6, and 9 h after PM treatment. b Relative transcript levels in leaves, stems, and roots at 6 h after PM treatment. c Relative transcript levels in 3-leaf seedlings after treatment with JA, SA, ABA, PEG4000, NaCl, or Cor. Controls were treated with distilled water. EF1α was detected as the internal control gene. Three biological replicates per treatment and three technical replicates were analyzed per sample. Significant differences are indicated by asterisks (**P < 0.01, *P < 0.05 by Student’s t-test), compared with the corresponding control. Error bars indicate the standard deviation of the mean
To compare CsDIR16-expression patterns in response to PM in different tissues, we analyzed leaves, stems, and roots at 6 h post-treatment in ‘D0351’ and ‘D9320’ plants (Fig. 5b). CsDIR16 was expressed in all 3 tissues, but the expression patterns differed between the 2 cucumber cultivars. CsDIR16 was upregulated by PM treatment in the leaves and stems of ‘D0351’. In contrast, CsDIR16 was expressed at extremely low levels in the leaves and stems of ‘D9320’. No statistically significant differences were observed in the roots of ‘D0351’ and ‘D9320’. These results indicated that CsDIR16 expression was significantly induced by PM treatment in the fruit, leaves, and stems of ‘D0351’, suggesting that these are the major organs for metabolism of the fungicide.
Expression pattern of CsDIR16 in response to hormone induction and various exogenous stresses
DIR genes are involved in various hormone responses and exogenous stresses [30,31,32,34]. In this research, the effects of other potential stressors on CsDIR16 expression were examined. The lowest PM-residue cultivar ‘D0351’ and the highest PM-residue cultivar ‘D9320’ were treated with jasmonic acid (JA), salicylic acid (SA), abscisic acid (ABA), polyethylene glycol 4000 (PEG4000), NaCl, or Cor. CsDIR16 expression in ‘D0351’ was significantly up-regulated by treatment with each compound, with expression levels 2.57, 7.40, 2.37, 4.00, 3.27, and 14.32-fold higher than those in the control (Fig. 5c), respectively. CsDIR16 expression in ‘D9320’ was significantly up-regulated by each compound, except for SA, and the relative fold-changes of expression were 3.02, 0.13, 4.24, 13.36, 5.44, and 16.88-fold higher, respectively, than that in the control (Fig. 5c). These results revealed that CsDIR16 could be up-regulated by JA and ABA, indicating that CsDIR16 may be involved in JA- and ABA-associated signaling pathways. The expression pattern after SA treatment was significantly higher in ‘D0351’ compared to ‘D9320’, reflecting the same pattern observed after PM treatment. These results indicated that CsDIR16 expression was significantly induced by JA, SA, ABA, PEG4000, NaCl, and Cor stress, suggesting that CsDIR16 might be involved in cultivar responses to various biological and abiotic stresses.
CsDIR16 overexpression enhanced PM metabolism in transgenic cucumbers
The over-expression vectors CsDIR16(+)-PCXSN and CsDIR16(−)-PCXSN were successfully transferred into the highest PM-residue cultivar D9320 using cucumber genetic-transformation technology (Fig. 6). We generated transgenic cucumbers overexpressing CsDIR16 under the control of the strong constitutive CaMV35S promoter (Fig. 7a). Transgenic cucumbers T0 and T1 were identified by PCR (Fig. 7b), and the expression of CsDIR16 was analyzed by quantitative real-time PCR (qRT-PCR; Fig. 7c). Both T0 and T1 transgenic cucumbers showed increased levels of CsDIR16 expression (approximately 3-fold higher). In T0 and T1 CsDIR16(−) transgenic cucumbers, the level of expression was approximately 0.4 that of the normal control. Overall, we obtained 13 CsDIR16(+) and 15 CsDIR16(−) T0 transgenic cucumbers.
Characterization of CsDIR16-overexpression transgenic plants. a Schematic representation of the CsDIR16-overexpression plasmid. b Transgenic plants T0 and T1 were identified by PCR. c Relative CsDIR16 transcript levels in transgenic plants T0 and T1. Error bars indicate the standard deviation of the mean. Superscripted letters represent significant differences at the 0.05 level, based on Tukey’s test
We compared the levels of PM residues in wild-type ‘D9320’, and T0 and T1 CsDIR16(+) and CsDIR16(−) transgenic cucumbers. The level of residual PM was lower in CsDIR16(+) transgenic plants than in wild-type ‘D9320’ and CsDIR16(−) plants after PM treatment (Fig. 8a and b). The PM levels did not differ significantly between wild-type ‘D9320’ and CsDIR16(−) transgenic plants. As CsDIR16 was not a main gene responsible for metabolic removal of PM in ‘D9320’, the presence of the antisense expression vector CsDIR16(−) did not affect PM metabolism in CsDIR16(−) transgenic cucumbers. Our results indicated that CsDIR16 overexpression is likely to reduce PM residue levels by accelerating degradation of the fungicide.
Quantitative analysis of propamocarb (PM) residues, peroxidase (POD) activity, and the malondialdehyde (MDA) content in transgenic plants. a Quantitative analysis of PM residues in T0 transgenic plants using gas chromatography. b Quantitative analysis of PM residues in T1 transgenic plants using gas chromatography. c Quantitative analysis of POD activity in T1 transgenic plants. d Quantitative analysis of the MDA content in the T1 transgenic plants. The means ± SD of 3 replicates are shown. Superscripted letters represent significant differences at the 0.05 level, based on Tukey’s test
CsDIR16 may require peroxidase (POD) activities to enhance PM metabolism in cucumbers
Pesticide molecules, just like toxic xenobiotics, could affect relevant enzyme activities and transform gene-expression patterns. Abiotic stresses in plants can cause excessive accumulation of reactive oxygen species (ROS). ROS are highly reactive and toxic that may lead to damage to proteins, lipids, carbohydrates, and DNA [31, 41]. Plants have defense mechanisms to protect against oxidative stress damage. POD is an antioxidant enzyme that scavenges ROS. Here, we measured POD activities after PM treatment in wild-type ‘D9320’, CsDIR16(+), and CsDIR16(−) T1 transgenic plants (Fig. 8c). After PM treatment, POD activity increased and peaked at 1 d in wild-type ‘D9320’ and CsDIR16(+) T1 transgenic plants. The CsDIR16(+) T1 transgenic cucumbers showed significantly more rapid reduction of POD activity compared with that in wild-type plants. CsDIR16(−) T1 transgenic line showed a similar trend to wild-type plants treated with water.
Malondialdehyde (MDA) is commonly used as a marker of oxidative lipid injury, whose concentration varies in response to biotic and abiotic stresses [42]. The MDA contents in wild-type ‘D9320’, CsDIR16(+), and CsDIR16(−) T1 transgenic cucumbers after PM treatment were determined (Fig. 8d). We found that MDA contents increased significantly and reached a peak at 9 h in the CsDIR16(+) T1 transgenic line, but this peak was only reached at 3 d after PM treatment in the wild-type ‘D9320’ cultivar and the CsDIR16(−) T1 transgenic line. After 9 h, the MDA content in CsDIR16(+) T1 transgenic plants decreased more rapidly compared with wild-type plants and CsDIR16(−) T1 transgenic plants. These results suggested that CsDIR16 overexpression reduced PM residues through accelerating PM degradation.
Discussion
Many plant genes are induced by biological and abiotic stresses, such as insects, fungi, drought, and high-salinity. Not only may these genes function in stress responses, but in stress tolerance as well [43, 44]. Gene-expression patterns are often associated with gene function [24]. In a previous study, the transcriptome changes in cucumber fruit of cultivar ‘D0351’ in response to PM treatment were analyzed by our research group. [21]. The transcriptome data indicated that CsDIR16 showed the highest differential expression between plants treated with PM versus water. After treatment with PM, we found that only CsDIR16 showed significantly differential expression between ‘D0351’ and ‘D9320’ (Fig. 5a and b). CsDIR16 was significantly up-regulated in the fruits, leaves, and stems of the lowest-residue cultivar ‘D0351’. In the highest-residue cultivar ‘D9320’, CsDIR16 was expressed at extremely low levels in leaves and stems, with no significant up-regulation in fruits. The expression pattern of CsDIR16 was related to plant PM residues, which indicated that CsDIR16 plays an important role in the response to PM.
To investigate the role of CsDIR16 in PM responses, the CsDIR16 gene was transformed into the wild-type ‘D9320’ cultivar (Fig. 4a–d) and the effects of CsDIR16 overexpression on PM residues were measured. CsDIR16(+)-overexpressing transgenic cucumber plants showed lower PM residues and more rapid PM-residue reduction compared with wild-type and CsDIR16(−) plants (Fig. 8a and b). Dirigent proteins lack a catalytically active (oxidative) center and depend oxidases to biosynthesize lignin and lignan [35]. The 3-dimensional structure of CsDIR16 showed that it has a typical β-barrel structure with a hydrophobic chamber in the center (Fig. 2) and lacks an oxidative center.
POD is the key enzyme in the phenylpropanoid pathway. This pathway is significantly associated with the cucumber metabolism of PM [21]. POD activities increased in cultivar ‘D0351’ after treatment with PM, while lignin increased by 41.1% [45]. Here, we found that POD activity in CsDIR16(+) T1 transgenic plants fell more rapidly compared with that in wild-type plants, indicating that CsDIR16 could increase POD-activity responses to PM. The reduction in POD activity in CsDIR16(+) T1 transgenic plants followed the rapid reduction in PM residues. We found that the MDA content increased in wild-type ‘D9320’ after PM treatment, suggesting that the fungicide may cause damage to the plants. The MDA content fell more rapidly in CsDIR16(+) T1 transgenic plants compared with wild-type plants, indicating that the CsDIR16 gene can repair the plasma membrane and lead to a more rapid lowering of the MDA content. Based on these results, we speculate that PM activates the phenylpropanoid pathway, causing increased POD activity. According to this model, the phenoxy radicals scavenged by POD are oxidized by the CsDIR16 protein in the fruit, leaves, and stems to produce pinoresinol, which rapidly reduces the damage caused by PM by effectively diminishing the PM residues in ‘D0351’.
Phylogenetic analysis showed that CsDIR16 belongs to the DIR-b/d subfamily (Fig. 2a), which is responsive to abiotic stress. In our study, CsDIR16 was significantly up-regulated after JA, SA, ABA, PEG4000, NaCl, and Cor treatments (Fig. 5c), showing that it influences responses to such stressors [31, 40, 46, 47]. The phytohormone SA acts as a signaling molecule and can induce plant stress responses under adverse conditions. The similarity in the expression patterns of CsDIR16 (Fig. 5b and c) after SA or PM treatments in leaves of ‘D0351’ and ‘D9320’ may indicate that the regulatory mechanisms induced by SA are different in ‘D0351’ and ‘D9320’, resulting in different levels of PM residues.
Conclusion
In summary, the CsDIR16 protein localized to the cell nucleus and could respond to abiotic and biotic stresses. CsDIR16 adapted to PM treatment and reduce the levels of residues in CsDIR16(+) transgenic plants through enhancing plant metabolism and physiological functions. Further research on CsDIR16 function will provide additional genetic resources for breeding stress-resistant plants and may provide further insights into pesticide-stress mechanisms.
Methods
Plant materials and stress treatments
D0351 and D9320 used in this study were homozygous cucumber lines. The low-PM-residue cultivar ‘D0351’ and the high-PM-residue cultivar ‘D9320’ had been identified by Fangfang Liu [22]. The seeds were provided by cucumber research group of Northeast Agricultural University, Harbin, China. Seeds of ‘D0351’ and ‘D9320’ were germinated, and the seedlings were grown under following conditions: 25–30 °C day, 15–18 °C night; 60–75% relative humidity in a greenhouse at the College of Horticulture, Northeast Agricultural University, Harbin, China.
Young plants at 34 days after transplanting at the 3-leaf stage were used for expression analysis of CsDIR16 in response to PM treatment. 8 mM PM solution was sprayed to similar sizes plants for 1 min until the surface of the leaves and fruits began to drip [21, 23, 24]. The leaves, stems, roots, and fruit peels (ca. 2 mm thick, 1 cm2, from the nodes of 10 fruit per plant, respectively) were sampled at 0.5, 1, 3, 6, and 9 h after treatment. Control plants were sprayed with distilled water.
Three-leaf seedlings were used to determine changes in CsDIR16 expression after treatment with JA, SA, ABA, PEG4000, NaCl, and Cor. The plants were sprayed with 100 μmol/L JA, 100 μmol/L SA, or 100 μmol/L ABA [48]; leaves were harvested 12 h after treatment. Seedlings were irrigated with 50 mL 40% PEG4000; leaves were harvested 8 days after treatment. Seedlings were irrigated with 50 mL 400 mmol/L NaCl, once every 3 days; leaves were harvested 8 days after treatment. Seedlings were sprayed with 1 × 105 colony-forming units/mL Cor; leaves were harvested 24 h after treatment. All samples were immediately frozen in liquid nitrogen and stored at − 80 °C until used for RNA extraction.
To measure the effects of CsDIR16(+) and CsDIR16(−) overexpression on PM residues, T0 transgenic cucumber young plants with similar levels of CsDIR16 expression were sprayed with 8 mM PM solution. Leaves were harvested at 0.5, 1, 3, 6, and 9 h after treatment. Wild-type ‘D9320’ plants were sprayed as controls. Seeds of the T1 generation were obtained via the self-cross of the T0 generation lines. Three-leaf T1 transgenic cucumber seedlings with similar levels of CsDIR16 expression were sprayed with 1 mM PM solution. Leaves were harvested at 1 h, 3 h, 6 h, 9 h, 1 d, 3 d, 5 d, and 7 d after treatment. Wild-type ‘D9320’ plants were sprayed as controls.
Gene cloning and bioinformatics analysis of CsDIR16
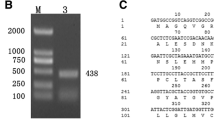
Total RNAs (from leaves, stems, roots, and fruit peels) were extracted using the TRIzol reagent (Invitrogen). Total RNA (1 μg) was reverse transcribed with a ReverTra Ace qPCR RT Kit (Toyobo, Japan) for cDNA synthesis. The cucumber genome database was applied for searching the full-length coding sequences (CDS) of CsDIR16 gene (gene ID Csa4M280630.1), and Primer Premier 5.0 (PREMIER Biosoft International, CA, USA) was used to design the specific primers for cloning the full-length CDS. The full-length CsDIR16 ORF was amplified by PCR using the primers CsDIR-F (5′-ATGGCTGGAATCTCTCCAAT-3′) and CsDIR-R (5′-TCAATAATGAAGCACGTAAATGTTA-3′). PCR reaction was performed using the following thermocycling conditions: 94 °C for 5 min; followed by 31 cycles with 94 °C for 30s, 55 °C for 30s, 72 °C for 30s; and then 72 °C for 10 min. The amplicons were cloned into the pEASY-T3 vector (TransGen Biotech) and sequenced by GENEWIZ.
The deduced CsDIR16 protein sequence was analyzed using the Conserved Domain Database (CDD) of NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). DNAMAN software (http://www.lynnon.com/) was utilized to peform sequence alignments. A phylogenetic tree was constructed by MEGA7.0 software using neighbor-joining algorithm. All sequences data were obtained from NCBI (Additional file 2: Table S4).
Promoter sequence analysis
The promoter sequence, which was located 1410 bp upstream of the transcription start site, was obtained by a BLAST search of the cucumber genome database (http://www.icugi.org/). The online tool Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was used to analysis.
Subcellular-localization analysis
A CsDIR16-GFP (green fluorescent protein) vector was constructed by cloning the CsDIR16 ORF into a pGII-eGFP vector using the primers 5′-AACGGATCCATGGCTGGAATCTCTCCAAT-3′ (HindIII site underlined) and 5′-TCCCCCGGGAATAATGAAGCACGTAAATGTTA-3′ (SmaI site underlined). The plasmids pGII-eGFP and pGII:CsDIR16-eGFP were transformed into Arabidopsis protoplast cells [24]. Subcellular localization in protoplasts was observed using a TCS SP2 confocal spectral microscope imaging system (Leica, Germany).
qRT-PCR analysis
Total RNA was extracted and subjected to reverse transcription as described above. qRT-PCR was performed using SYBR® Green Realtime PCR Master Mix (Toyobo, Japan) in an iQ5 (Bio-Rad) thermocycler. The amplification conditions were as follows: denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 55 °C for 15 s. Relative quantitation of gene expression was performed using CsEF1α (GenBank Accession Number: XM_004138916) as control [43]. Four replicates were used for each treatment. Melting-curve analysis was performed after the amplification was complete. The 2-ΔΔCT method was used for analyzing the real-time qPCR results.
The following gene-specific primers were used: CsDIR-qF (5′-ATAGCCGAAGATGAAAACTCCT-3′) and CsDIR-qR (5′-TTGGACCGCACCGAATC-3′); EF1α-qF (5′-CCAAGGCAAGGTACGATGAAA-3′) and EF1α-qR (5′-AGAGATGGGAACGAAGGGGAT-3′).
Expression vector construction and transformation of cucumbers
The plant expression vector pCXSN was used for TA cloning. The T-DNA region selection markers for hygromycin resistance were replaced by the herbicide-resistance gene bar [49, 50]. There were two XcmI restriction sites downstream of CaMV35S promoter in pCXSN. The over-expression vector was constructed by the RT-PCR products of CsDIR16 ligating into the pCXSN vector, which had been digested with XcmI (Additional file 1: Figure S4). An overexpression vector, CsDIR16(+)-PCXSN, was constructed through TA cloning, as well as an antisense expression vector, CsDIR16(−)-PCXSN. The directionality of the target gene within the vector was confirmed by sequencing using the primers pCXSN-F (5′-CGGCAACAGGATTCAATCTTA-3′) and pCXSN-R (5′-CAAGCATTCTACTTCTATTGCAGC-3′).
The recombinant plasmids CsDIR16(+)-PCXSN and CsDIR16(−)-PCXSN were separately introduced into Agrobacterium tumefaciens strain LBA4404, then simultaneously transferred into ‘D9320’ cucumber cotyledons using the cucumber genetic-transformation system [48, 51], and tested for resistance to glufosinate (1 mg/L). PCR and qRT-PCR analyses were performed on the transgenic plants.
Measurements of PM residues, POD activity, and MDA content
The level of PM residue was measured as described by Meng et al. [24]. Briefly, approximately 5.0 g of cucumber tissue was added to 25 mL of acetonitrile and homogenized with a high-speed homogenizer (Heidolph Silent Crusher-M®) for 2–3 min at 14–15000×g, and stood at room temperature for 1 h. 3 g NaCl was added into each extraction, then vortexed vigorously for 1 min and centrifuged for 10 min at 5000×g. 5 mL of each supernatant was dried with Termovap sample concentrator. 1 mL methyl alcohol was added to the residues, and then filtered through a 0.22-μm polypropylene filter. Agilent 7890A gas chromatography system (Agilent Technologies) equipped with a capillary column (HP-5, 30 m × 0.25 mm × 0.25 μm) was applied to analyze the level of PM residue. The column temperature was sustained 40 °C for 2 min, and then raised to 200 °C at the speed of 25 °C·min− 1, and held at that temperature for 8 min. Nitrogen was used as the carrier gas, with a hydrogen flow rate of 60 ml·min− 1, an air flow rate of 400 ml·min− 1, and a tail wind flow rate of 60 ml N2·min− 1. The injection port temperature and detection temperature were both set at 240 °C.
To determine the activities of POD enzymes, fresh leaf material (500 mg) was homogenized in 5 ml of 50 mmol phosphate buffer (pH = 7.0) containing 1% soluble polyvinylpyrrolidone. The homogenates were centrifuged at 15000×g for 10 min, and the POD activities in the supernatants were determined spectrophotometrically by measuring the absorbance at 470 nm, as described previously [52]. The reaction mixture contained 5 × 10− 3 M guaiacol and 5 × 10− 3 M H2O2 in 0.1 M phosphate buffer (pH = 6.0). The reaction was initiated by adding 20 μL of protein extract to 3 mL of reaction mixture. Changes in absorbance, due to the catalytic conversion of pyrogallol to purpurogallin, were measured at 30-s intervals for 3 min at 470 nm.
MDA levels were measured as described by Wu et al. [53]. The thiobarbituric acid (TBA) method was used to detect the amount of MDA. Briefly, approximately 0.5 g of sample was homogenized with 5 mL 10% trichloroacetic acid (TCA). The homogenate was centrifuged at 11,000×g for 15 min. 1 mL supernatant was mixed with 2 mL 10% TCA containing 0.67% TBA. The mixture was heated in boiling water bath for 15 min, then cooled immediately in an ice bath, and centrifuged at 4000×g for 20 min. The absorbance values of the supernatant at 600, 532, and 450 nm were determined with ultraviolet–visible spectrophotometer (Shimadzu, Japan). The quantity of MDA was calculated using the following equations:
Statistical analysis
All data measurements were replicated at least 3 times. The data were subjected to statistical analyses using the Origin8.0, DPS7.05 (Data Processing System), and GraphPad Prism 6 programs. Data were expressed as the mean ± SD. Significant differences between the treatment and control groups were confirmed by Student’s t-tests. The data were analyzed by analysis of variance (ANOVA; p < 0.001), followed by Tukey’s test to compare differences between the groups at a significance level of p < 0.05.
Abbreviations
- ABA:
-
abscisic acid
- cDNA:
-
complementrary DNA
- CDS:
-
coding sequences
- Cor:
-
Corynespora cassiicola Wei
- DIR:
-
dirigent protein
- GFP:
-
green fluorescent protein
- JA:
-
jasmonic acid
- PCR:
-
polymerase chain reaction
- PEG4000:
-
Polyethylene glycol 4000
- PM:
-
propyl-[3-(dimethylamino) propyl]carbamate
- qRT-PCR:
-
real-time quantitative reverse transcription-polymerase chain reaction
- RNA:
-
ribonuncleic acid
- RT-PCR:
-
reverse transcription-polymerase chain reaction
- SA:
-
salicylic acid
- Tag-Seq:
-
high-throughput tag-sequencing
References
Zhou Y, **a X, Yu G, Wang J, Wu J, Wang M, et al. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci Rep. 2015;5:9018.
Saravi SS, Shokrzadeh M. Effects of washing, peeling, storage, and fermentation on residue contents of carbaryl and mancozeb in cucumbers grown in greenhouses. Toxicol Ind Health. 2016;32:1135–42.
Leili M, Pirmoghani A, Samadi MT, Shokoohi R, Roshanaei G, Poormohammadi A. Determination of pesticides residues in cucumbers grown in greenhouse and the effect of some procedures on their residues. Iran J Public Health. 2016;45:1481–90.
Pretali L, Bernardo L, Butterfield TS, Trevisan M, Lucini L. Botanical and biological pesticides elicit a similar induced systemic response in tomato (Solanum lycopersicum) secondary metabolism. Phytochemistry. 2016;130:56–63.
Mahboob S, Niazi F, Alghanim K, Sultana S, Almisned F, Ahmed Z. Health risks associated with pesticide residues in water, sediments and the muscle tissues of Catla Catla at head Balloki on the river Ravi. Environ Monit Assess. 2015;187:81.
Cremonese C, Piccoli C, Pasqualotto F, Clapauch R, Koifman RJ, Koifman S, et al. Occupational exposure to pesticides, reproductive hormone levels and sperm quality in young Brazilian men. Reprod Toxicol. 2017;67:174–85.
Clara V, Nieto R, Rosa M, Nadia B, Victoria LL, Horacio R, et al. Pesticide chlorpyrifos acts as an endocrine disruptor in adult rats causing changes in mammary gland and hormonal balance. J Steroid Biochem Mol Biol. 2015;156:1–9.
Farivar TN, Peymani A, Najafipour R, Mehr MA, Alizadeh S, Johari P. Biodegradation of paraoxan as an organophosphate pesticide with Pseudomonas plecoglocissida transfected by opd gene. Biotech Health Sci. 2017;4:e13435.
Mehr MA, Farivar TN, Najafipour R, Peymani A, Alizadeh S, Johari P. Biodegradation of endosulfan as an organochlorine pesticide with Pseudomonas plecoglocissida transfected by LinA gene. Biotech Health Sci. 2017; https://doi.org/10.5812/bhs.45306.
Oerke EC, Steiner U, Dehne HW, Lindenthal M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J Exp Bot. 2006;57(9):2121–32.
Elliott M, Shamoun SF, Sumampong G. Effects of systemic and contact fungicides on life stages and symptom expression of Phytophthora Ramorum Invitro and in planta. Crop Prot. 2015:67136–44.
Taylor JC, Hird SJ, Sykes MD, Startin JR. Determination of residues of Propamocarb in wine by liquid chromatography-electrospray mass spectrometry with direct injection. Food Addit Contam. 2004;21(6):572–7.
Hiemstra M, Kok AD. Determination of Propamocarb in vegetables using polymer-based high-performance liquid chromatography coupled with electrospray mass spectrometry. J Chromatogr A. 2002;972(2):231–9.
Sahoo SK, Battu RS, Singh B. Development and validation of Quechers method for estimation of Propamocarb residues in tomato (mill) and soil. Am J Anal Chem. 2011; https://doi.org/10.4236/ajac.2011.228120.
Abd-Alrahman SH, Almaz MM. Degradation of Propamocarb-hydrochloride in tomatoes, potatoes and cucumber using HPLC–DAD and QuEChERS methodology. Bull Environ Contam Toxicol. 2012;89(2):302–5.
Mpina MH, Hosea F. Fenamidone + propamocarb hydrochloride: a promising package for the control of early and late blights of tomatoes in northern tanzania. Int J res Agr Forestry. 2016;3(3):1–7.
Schmuck G, Mihail F. Effects of the carbamates Fenoxycarb, Propamocarb and Propoxur on energy supply, glucose utilization and Sh-groups in neurons. Arch Toxicol. 2004;78(6):330–7.
Aydemir N, Bilaloğlu R. The investigation of the genotoxic effects of Fenarimol and Propamocarb in mouse bone marrow in vivo. Toxicol Lett. 2004;147(1):73–8.
Liu D, **n M, Zhou X, Wang C, Zhang Y, Qin Z. Expression and functional analysis of the transcription factor-encoding gene CsERF004 in cucumber during Pseudoperonospora cubensis and Corynespora cassiicola infection. BMC Plant Biol. 2017;17:96.
Holmes GJ, Ojiambo PS, Hausbeck MK, Quesada-Ocampo L, Keinath AP. Resurgence of cucurbit downy mildew in the United States: a watershed event for research and extension. Plant Dis. 2015;99:428–41.
Wu P, Qin Z, Zhao W, Zhou X, Wu T, **n M, et al. Transcriptome analysis reveals differentially expressed genes associated with propamocarb response in cucumber (Cucumis sativus L.) fruit. Acta Physiol Plant. 2013;35:2393–406.
Liu F, Qin Z, Zhou X. Screening germplasm resources of cucumber plant with low pesticide residue content. J Northeast Agr Univ. 2010;41:32–6.
Wu P. Studies on physiological and molecular basis of low propamocarb residue in cucumber. Har bin: Northeast Agricultural University; 2013.
Meng J-J, Qin Z-W, Zhou X-Y, **n M. An ATP-binding cassette transporter gene from Cucumis Sativus L., Csabc19, is involved in propamocarb stress in Arabidopsis thaliana. Plant Mol Biol Report. 2016;34:947–60.
Li S, Qin Z, **n M, Zhou X. Expression and Functional analysis of Cswrky30 in cucumber under propamocarb stress. Sci Agric Sin. 2016;49:1277–88.
Davin LB, Wang H-B, Crowell AL, Bedgar DL, Martin DM, Sarkanen S, et al. Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science. 1997;275:362–7.
Gang DR, Costa MA, Fujita M, Dinkova-Kostova AT, Wang H-B, Burlat V, et al. Regiochemical control of monolignol radical coupling: a new paradigm for lignin and lignan biosynthesis. Chem Biol. 1999;6:143–51.
Burlat V, Kwon M, Davin LB, Lewis NG. Dirigent proteins and dirigent sites in lignifying tissues. Phytochemistry. 2001;57:883–97.
Dalisay DS, Kim KW, Lee C, Yang H, Rübel O, Bowen BP, et al. Dirigent protein-mediated lignan and cyanogenic glucoside formation in flax seed: integrated omics and MALDI mass spectrometry imaging. J Nat Prod. 2015;78:1231–42.
Rang Z, Zhou Q. Bioinformatic analysis of the dirigent gene family in rice. J Hunan Agri Univ (Nat Sci). 2013;2:111–20.
Wu R, Wang L, Wang Z, Shang H, Liu X, Zhu Y. Cloning and expression analysis of a dirigent protein gene from the resurrection plant Boea hygrometrica. Prog Nat Sci. 2009;19:347–52.
Guo J. Quantitative RNA-Seq analysis of the sugarcane response to peg and the identification of differentially expressed genes: Fujian Agriculture and Forestry University; 2013.
Zhao F, Fang W, Yang X, **e D, Li W, Tang Z. Cloning and analysis of upland cotton (Gossypium hirsutum) dirigent-like gene (GhDIR). Acta Agriculturae Boreali-Sinica. 2011;5:29–33.
**-long G, Li-** X, **g-** F, Ya-chun S, Hua-ying F, You-xiong Q, et al. A novel dirigent protein gene with highly stem-specific expression from sugarcane, response to drought, salt and oxidative stresses. Plant Cell Rep. 2012;31:1801–12.
Davin LB, Lewis NG. Lignin primary structures and dirigent sites. Curr Opin Biotechnol. 2005;16:407–15.
Pickel B, Pfannstiel J, Steudle A, Lehmann A, Gerken U, Pleiss J, et al. A model of dirigent proteins derived from structural and functional similarities with allene oxide cyclase and lipocalins. FEBS J. 2012;279:1980–93.
Li Q, Chen J, **ao Y, Di P, Zhang L, Chen W. The dirigent multigene family in Isatis Indigotica: gene discovery and differential transcript abundance. BMC Genomics. 2014;15:388.
Ma Q-H, Liu Y-C. TaDIR13, a dirigent protein from wheat, promotes lignan biosynthesis and enhances pathogen resistance. Plant Mol Biol Report. 2015;33:143–52.
Halls SC, Davin LB, Kramer DM, Lewis NG. Kinetic study of coniferyl alcohol radical binding to the (+)-pinoresinol forming dirigent protein. Biochemistry. 2004;43:2587–95.
Thamil Arasan SK, Park JI, Ahmed NU, Jung HJ, Hur Y, Kang KK, et al. Characterization and expression analysis of dirigent family genes related to stresses in Brassica. Plant Physiol Biochem. 2013;67:144–53.
Erinle KO, Zhao J, Ma B, Li J, Chen Y, Ur-Rehman K, et al. Exogenous calcium induces tolerance to atrazine stress in Pennisetum seedlings and promotes photosynthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicol Environ Safety. 2016;132:403–12.
Davey M, Stals E, Panis B, Keulemans J, Swennen R. High-throughput determination of malondialdehyde in plant tissues. Anal Biochem. 2005;347(2):201–7.
Zhai Y, Zhang L, **a C, Fu S, Zhao G, Jia J, et al. The wheat transcription factor, TabHLH39, improves tolerance to multiple abiotic stressors in transgenic plants. Biochem Biophys Res Commun. 2016;473:1321–7.
Liu Y, Zhang X, Zhu S, Zhang H, Li Y, Zhang T, et al. Overexpression of GhSARP1 encoding a E3 ligase from cotton reduce the tolerance to salt in transgenic Arabidopsis. Biochem Biophys Res Commun. 2016;478:1491–6.
Wu P, Guo QQ, Qin ZW. The fungicide propamocarb increases lignin by activating the phenylpropanoid pathway in Cucumis sativus L. Hortic Environ Biotechnol. 2016;57:511–8.
Behr M, Legay S, Hausman JF, Guerriero G. Analysis of cell wall-related genes in organs of Medicago sativa L. under different abiotic stresses. Int J Mol Sci. 2015;16:16104–24.
Liao Y, Liu S, Jiang Y, Hu C, Zhang X, Cao X, et al. Genome-wide analysis and environmental response profiling of dirigent family genes in rice (Oryza sativa). Genesn. 2017;39:47–62.
Li S. Functional analysis of CsWRKY30 gene in cucumber under propamcarb stress. Harbin: Northeast Agricultural University; 2016.
Chen S, Songkumarn P, Liu J, Wang GL. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009;150(3):1111.
Li M, Tan W, Sun M, Li Y, Li W. Construction of TA cloning plant expression vector with herbicide resistance. Genomics Appl Biol. 2015;11:2436–40.
Zhang Y, Zhang X, Liu B, Wang W, Liu X, Chen C, et al. A Gamyb homologue CsGAMYB1 regulates sex expression of cucumber via an ethylene-independent pathway. J Exp Bot. 2014;65:3201–13.
Lepeduš H, Gaća V, Cesar V. Guaiacol peroxidases and photosynthetic pigments during maturation of spruce needles. Croatica Chemic Acta. 2005;78(3):355–60.
Peng WU, Qin ZW, Tao WU, Zhou XY, **n M, Guo QQ. Proteomic analysis of cucumber defense responses induced by propamocarb. J Integr Agric. 2013;12:2022–35.
Acknowledgements
We acknowledge associate Professor Yongguang Li (Key Laboratory of Northeastern Soybean Biology and Genetic Breeding of the Ministry of Agriculture, China) for providing the pCXSN vector. We thank Professor Huazhong Ren (College of Agronomy and Biotechnology, China Agricultural University, Bei**g) for providing the method for genetic transformation of cucumber.
Funding
The design of study and collection of samples were funded by The National Natural Science Foundation of China (31272158). The analysis and interpretation of data were financially supported by the National Natural Science Foundation of China (31401863). The regents and supplies financially supported by the Young University Innovative Talent Training Program of Heilongjiang Province (UNPYSCT-2016007).
Availability of data and materials
The data sets supporting the results of this article are included within the article and additional file. Materials are available by contacting the corresponding author. Bioinformatics data are provided in Fig. 1, Additional file 1: Figure S2 and Figure S3. Data on the three-dimensional structure of the CsDIR16 protein, from different views are in Fig. 2. Data on phylogenetic analysis and sequence alignment of CsDIR16 are in Fig. 3, Additional file 1: Figure S1, Tables S2 and S3. Data on subcellular localization are in Fig. 4. Data on expression pattern after PM treatment and different stresses are in Fig. 5. Data on pCXSN-CsDIR16(±) genetic transformation are in Fig. 6 and Additional file 1: Figure S4. Data on the characterization of CsDIR16-overexpression transgenic plants are in Fig. 7. Data on the content of PM residues, POD and MDA in transgenic plants are in Fig. 8. Data on the cis elements in the CsDIR16 promoter are provided in Additional file 1: Table S1. All sequences data in Fig. 3 are in Additional file 2: Table S4.
Author information
Authors and Affiliations
Contributions
CHL, ZQ, XZ and MX designed experiments. CHL performed the following experiments: expression pattern analysis, transformation of cucumber, determination of PM residues, peroxidase activity and malondialdehyde content. CW and SL prepared the plant materials, performed RNA extractionand and the subcellular localization. CHL wrote the manuscript and analyzed the data. ZQ revised the manuscript critically for important intellectual content. DL did the analysis and interpretation of data and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Figure S1.
Amino acid sequence alignment of the DIR family from cucumber, Arabidopsis thaliana (At) and Cucumis melo (Cm). Alignment generated using ClustalW (blosum matrix, gap open and gap extension penalties of 5 and 1.0, respectively) and Boxshade. Conserved similarity shading is based on 50% identity (black) and 50% similarity (gray). Figure S2. Signal peptide prediction of CsDIR16 coding protein Figure S3. Transmembrane analysis of CsDIR16 coding protein, Figure S4. Construction of plant vector CsDIR16-pCXSN(±), Table S1. Locations and sequences of cis-elements in the promoter regions of the CsDIR16 genes, Table S2. Identified CsDIR genes in cucumber genome, Table S3. CsDIRs gene that responds to PM stress. (DOCX 433 kb)
Additional file 2: Table S4.
All sequences data in Fig. 2. (DOCX 26 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, C., Qin, Z., Zhou, X. et al. Expression and functional analysis of the Propamocarb-related gene CsDIR16 in cucumbers. BMC Plant Biol 18, 16 (2018). https://doi.org/10.1186/s12870-018-1236-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-018-1236-2