Abstract
Background
Ganzi Prefecture in Western China is situated geographically at the transition regions between Tibetan Plateau and Sichuan Basin in a highly tectonically active boundary area between the India and Eurasia plates. The region hosts various hot springs that span a wide range of temperature from 30 to 98 °C and are located at high altitude (up to 4200 m above sea level) in the region of large geothermal anomalies and active ** bacterial community structure of hot springs as revealed by correlation analysis. Abundant unassigned-genus sequences detected in this study strongly implied the presence of novel genera or genetic resources in these hot springs.
Conclusion
The diversity of hot springs of Ganzi prefecture in Western Sichuan, China is evidently shaped by temperature. Interestingly disproportionally abundant unassigned-genus sequences detected in this study show indicate potential of novel genera or phylotypes. We hypothesize that frequent earthquakes and rapidly changing environment might have contributed to evolution of these potentially new lineages. Overall, this study provided first insight into the bacterial diversity of hot springs located in Western Sichuan, China and its comparison with other similar communities worldwide.
Similar content being viewed by others
Background
Microbial populations in terrestrial hot springs have been a subject of interests and concerns of microbiologists. The hot springs around the world have been extensively studied, particularly in Yellowstone National Park (YNP), USA [1], Japan [2], United States Great Basin [3], Iceland [4], and Malaysia [5]. These sites possess diverse physicochemical properties and biological species. In China, similar studies also have been conducted on hot springs, primarily at Tengchong County, Yunnan Province [6, 7] and Tibetan Plateau [8]. Many springs at Tengchong harbored similar microbial communities at the phylum and family/genus levels, as compared to springs at Yellowstone National Park, probably due to the fact that both were volcanically driven with acidic springs [6]. Diverse microorganisms were also documented in Tibetan hot springs, including archaeal phyla Crenarchaeota, Euryarchaeota, and Thaumarchaeota, and various bacterial phyla such as Cyanobacteria, Chloroflexi, Chlorobi, Proteobacteria, and Firmicutes [8]. Although similarities could be found between different geographical fields, the microbial composition usually differs due to the great impact of physicochemical conditions, such as temperature and pH, on microbial diversity and distribution [3, 6]. Therefore, unique microbial communities are often shaped by distinct niches.
Ganzi Prefecture is located in Western Sichuan Province of China and geographically at the transition regions between Tibetan Plateau and Sichuan Basin. Tectonically, Ganzi is situated at the collision boundary between the India and Eurasia plates [9]. Active new tectonic movement resulted in the abundant hot springs throughout the Ganzi prefecture [9]. The hot springs in Ganzi span a wide range of temperature from 30 to 98 °C and are located at high altitude (up to 4200 m above sea level) [10] in the region of large geothermal anomalies and ** bacterial community structure in hot springs. However, no significant correlation was observed between pH (R2 = 0.10) or altitude (R2 = 0.20) and bacterial community structure.
Discussion
In the present study, the bacterial communities of 14 hot springs distributed in Western Sichuan, China, were investigated for the first time through molecular examination. Different analyses were conducted to elucidate the diversity of each hot spring. All clustering analysis supported to categorize the 14 spring samples into four bacterial community groups. Correlation between geochemical parameters and bacterial community structure was also studied.
The Alpha diversity analysis revealed that the values of four indices greatly varied among spring samples (Table 2). This finding could be explained mostly by the distinct temperature between the springs (Table 1). It is known that temperature is an important determinant of bacterial diversity [5]. Normally, microbial richness/diversity showed a positive or negative liner correlation with temperature [2, 36]. Such linear correlation, however, was not detected in this study, what has been also observed in many studies before [36,37,38]. These results indicate that although very important, temperature is not an exclusive determinant of microbial diversity in these hot springs. A remarkable decline in species richness was observed when the spring temperature exceeded 65 °C. Simultaneously, with an increase of temperature cyanobacteria become less abundant, green microbial mats were absent and typical hyperthermophilic organisms such as Aquificae and certain thermophilic members of Proteobacteria became more abundant. The disappearance of cyanobacteria, especially above 75 °C, is in agreement with previous studies that indicate that oxygenic photosynthesis is possible up to approximately 73–75 °C [39]. Above these temperatures, even the most thermophilic cyanobacteria described to date i.e. Synechococcus sp. OH28 isolated from Hunter’s Hot Springs in Oregon, USA [39] cannot actively grow. Although one of the springs indicated in this study ZG-4 (85.0 °C, pH 8.5) shows significant contribution of cyanobacterial population to the overall community structure, it is likely the result of sequencing approach used in the study. The 16S PCR products originating from DNA samples isolated from sediments and microbial mats collected from different parts of the spring were mixed together before sequencing. The cyanobacterial sequences are likely to originate from cooler parts of the spring and it is those sequences that contributed cyanobacterial part of the diversity. This has been confirmed by our parallel culture-dependent study that showed that although strains isolated from ZG-4 (equivalent to B4 strain series in that study) were among the most thermophilic ones and capable of growth at 60 °C they were unable to actively grow at temperatures higher than 65 °C [17].
An exception to the overall trend of decreasing microbial diversity as a response to increase of spring temperature is spring LL-2 (75.2 °C, pH 8.41) containing numerous reddish microbial mats. Detailed comparative analysis of the community composition of this spring is problematic due to very high percentage (73.16%) of unassigned at genus level sequences. At phylum level the spring is mainly composed of Proteobacteria (42.39%), Chloroflexi (13.51%), and Bacteroidetes (9.72%). Comparison of LL-2 spring with other hot springs of similar temperature and pH reveals both similarities and differences. The community structure of LL-2 is most similar to the recently described Mihai-Bravu (MB) spring in Romania (65 °C, pH 7.91) [40]. The MB spring sampled at 65 °C was dominated by Proteobacteria, Aquificae and Chloroflexi with minor contribution of Bacteroidetes making the community similar to that of LL-2. These results suggest that these communities may be sustained by anoxygenic photosynthesis, oxidation of hydrogen or combination of both factors [40].
One of the springs that has similar parameters to LL-2 is bicarbonate-chloride-fluoride low mineral-type Lobios hot spring (76 °C, pH = 8.2) located in Ourense (Spain) [41]. The spring is predominantly composed of bacteria: Deinococcus-Thermus, Proteobacteria, Firmicutes, Acidobacteria, Aquificae, and Chloroflexi. This alkaline spring is therefore inhabited by thermophilic, neutrophilic, and alkaliphilic organisms which belong to both aerobes and anaerobes with the largest abundance of Thermus species. It therefore shows different community structure to LL-2. Another spring similar in pH and temperature described in the literature is a sulfate-bicarbonate spring in Rupi Basin, Bulgaria [42]. The temperature and pH of the spring were 79 °C and 8.6, respectively. This spring was dominated by Proteobacteria (28.8%), Hydrogenobacter/Aquifex group (26.3%) and Deinococcus-Thermus (19.5%). Community structure of the Bulgarian spring suggested that metabolic activities are likely to be distinct of those of LL-2. Rupi Basin spring is likely to be shaped through co-existence of hydrogen-oxidizing Hydrogenophilus and Hydrogenobacter/Aquifex with hydrogen producing Thermus and Thermotoga, indicating hydrogen metabolism as an important component of that ecosystem. A similar type of microbial community was present in sulfuric hot spring Scary Spring (pH 7.8, 75 °C), Calcite Springs, YNP. Scary Spring has a very simple community structure composed predominantly of Thermus aquaticus (45%) and Sulfurihydrogenibium sp. (36%) [43], largely distinct than that of LL-2. One of three alkaline springs from Heart Lake Geyser Basin, Western Subgroup in YNP, that have similar basic parameters (pH 8.5, 75 °C) to LL-2 have been also assessed for their community structure [44] and showed to be different from that of Lotus lake spring. The microbial community of the spring is largely dominated by Thermus, analogously to the previous springs described above. In China, an alkaline spring Shuirebaozha in Tengchong region, Yunnan has most similar pH and temperature (pH 8.28, 78.2 °C) to LL-2 [6]. The bacterial community in the spring is dominated by Aquificae with secondary contributions from Chloroflexi and Thermotogae indicating very distinct composition from that of LL-2. All of the springs described in this section have lower species richness and diversity indices than that of LL-2.
To summarize, LL-2 has largely atypical community structure, and unusual biodiversity when compared to other springs with similar temperature and pH worldwide. The reasons of that can be many-fold and there is not enough evidence in the current dataset to make final conclusions. Some of the possible explanations are presented below. Different mineralogy to other springs in the area; it has been shown that hot springs even in close proximity can significantly differ in mineralogy, pH and temperature [6]. Water flows and temperature change patterns; unlike well-known springs of YNP and Tengchong, and even Danba (DB) or Erdaoqiao (ED) presented in this study Lotus Lake springs are remote and largely unknown to scientific community and only visited by autochthonic Tibetan peoples. The water flow patterns and temperature changes are largely unknown so one cannot accurately describe the history of the spring. We take into consideration the fact that the spring may experience a degree of temperature fluctuations that result in formation of more diverse community than typically found for springs of this temperature. Both temperature and flow pattern fluctuations have been reported for hot springs previously and are considered as important contributors to biodiversity and community structure [40, 45]. Another possibility is the carryover of non-endemic material e.g. soil from outside the pool to the pool itself. Since genomic DNA was used as a template for 16S rRNA amplification in this study, there exists a possibility that even if the biological material cannot survive harsh conditions of the spring the genomic DNA can remain stable and yield over-representation of certain genera non-endemic to the spring. A study performed using both genomic DNA and RNA (cDNA) as a template for 16S amplification combined with soil sampling around the pool could be used to verify this possibility as proposed before [40]. Whilst all these hypotheses provide possible explanations of unusually high diversity of the spring they explain very little as to why almost three quarters (73.16%) of sequences originating from this spring cannot be classified at genus level. We believe that more detailed follow-up study of Lotus Lake springs will be required to solve at least some of these issues.
To summarize temperature effect on Ganzi hot springs, there is a non-monotonic relationship between microbial diversity and temperature of the springs assayed. Similar findings were reported in previous studies [8, 37, 46], and it is likely that albeit very important, the temperature is not a unique determinant of microbial diversity in the hot springs of Ganzi.
pH of the springs analyzed in this study is in relatively narrow pH range: from slightly acidic (ED, pH 6.32) to moderately alkaline (LL-3, pH 8.84). Extreme pH ranges are well known to significantly impact the biodiversity to the point where the biological niche is occupied only by several most adapted species. For example, acid mine drainage site in Iron Mountain, California, USA (pH 0.83, 42 °C) is inhabited by a very simple community dominated by an extremophilic Leptospirillum and Ferroplasma [47]. One of the best studied acidic hot springs are those of Diretiyanqu in Rehai, Tengchong, Yunnan, China [6]. Diversity of these springs is lower than those of more neutral pH and temperature dependent. For example, Diretiyanqu-1 spring (pH 2.58, 85.1 °C) is almost exclusively inhabited by archaeal strain belonging to Crenarchaeota, whilst colder Diretiyanqu-2 (pH 2.57, 64.5 °C) and Diretiyanqu-3 (pH 2.58, 55.1 °C) springs contain significant proportions of Aquificae and Proteobacteria. At alkaline pH range the effect is similar i.e. low biodiversity is observed only in large extremes, of both pH and temperature whilst moderately alkaline (pH 8.5–10) sites have comparable biodiversity to those of more neutral pH. For example, a study of hot springs in Jiemeiquan (pH 9.25, 93.6 °C) and Gumingquan (pH 9.36, 82.5 °C) in Rehai, Tengchong, Yunnan, China [6] revealed dominance of Aquificae over any other phyla. Typically lower temperature alkaline hot springs below 73 °C are typically dominated by cyanobacteria [39]. The decrease of microbial diversity is minor when compared with sites of neutral pH and similar temperature. Though no obvious correlation was observed between pH and diversity indices, previous studies implied that pH played important role in microbial diversity [5]. The effects are both direct and indirect since pH has an effect on mineralogy, acidic springs result in leaching metals like iron from the rocks [45] and alkaline springs affect the availability of bicarbonate for photosynthetic cyanobacteria [48]. The effect of pH on biodiversity of the hot springs is therefore more subtle than that of temperature. Although there is no statistical significance between diversity indices and pH, we have observed that these hot springs are hosts to some alkaliphilic cyanobacteria which can actively grow in pH exceeding 9.50 [17], which corresponds to the fact that geologically these hot spring are likely to be limestone-hosted [11]. Taken together, these results indicated that differences in richness/diversity could be ascribed to a combined impact of various environmental conditions, such as temperature, pH and mineralogy [6, 36].
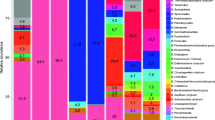
The bacterial communities in hot springs of Ganzi Prefecture were abundant in phyla Aquificae, Cyanobacteria and Proteobacteria (Fig. 2 and Additional file 4: Table S3). Although all the hot springs in this study were located at high altitude (2600–4200 m), the composition of bacterial community were common to that of low-altitude springs and does not appear to be unique to high-altitude springs. This result is consistent with previous finding in Tibetan springs (around 4500 m) [8, 37]. Differences in bacterial composition were also found between Ganzi hot springs and other geothermal springs. For instance, Thermodesulfobacteria were found to be abundant in springs of Tengchong, YNP and Great Basin [6, 49, 50]. However, low relative abundance of Thermodesulfobacteria was observed in Ganzi springs, with the exception of LL-7 where strong sulfur smell was observed (Table 1, Additional file 4: Table S3). This is probably because of the unfavorable geochemical conditions in Ganzi springs for Thermodesulfobacteria that showed the common presence in neutral pH (6.1–7.3) and high temperature (77–90 °C).
Within phyla Aquificae, Hydrogenobacter and Sulfurihydrogenibium were predominant genus. The two genera co-existed in several springs (Additional file 5: Table S4), which is consistent with observations in Tibetan and YNP hot springs under similar pH and temperature (i.e., near neutral to slightly alkaline pH and a temperature range of 60–80 °C) [8, 51]. Interestingly, the co-existence occurred in both sulfate pool (LL-7) and low-sulfate pool (LL-5) (Table 1). This result confirmed the previous finding that sulfide concentration is not a limiting factor for the co-existence of the two genera [8]. Unexpectedly, the co-existence was also noticed in spring LL-1 (Additional file 5: Table S4) which has a high temperature of 95 °C and high pH of 8.3. The possible reason may be that microorganisms in the spring may have distinct physiological properties. Additionally, the prominent Aquificae in springs indicated that chemolithotrophy by oxidation of H2, reduced sulfur compounds (sulfur or sulfide), or one-carbon compounds (formate or formaldehyde) are likely important metabolic processes in these springs [6].
Cyanobacterial sequences were abundant in spring ED, ZG-4 and DB (Fig. 2) and were dominated (72.37–99.67%) by members that could not be assigned below the class level (Additional file 5: Table S4). This result indicated the presence of unique members within the cyanobacterial taxonomy and yet unknown ecological role of these prokaryotic members. This is confirmed by our recent culture-dependent study of cyanobacteria from these springs where four out of five potential novel phylotypes of Leptolyngbyaceae were identified, the fifth one being LL [17]. Spring LL-4 was also abundant in cyanobacterial sequences, majority of which (81.13%) were affiliated with family GpXIII (Additional file 5: Table S4). The other cyanobacterial sequences detected in this study were related to groups (GpI, GpIIa, GpIV, GpV, GpVI, GpIX and GpXI) and families (Bacillariophyta, Chlorophyta and Streptophyta) (Additional file 5: Table S4). The classification scheme used in this study followed the RDP classifier [52], which is based on the classification described in Bergey’s Manual of Systematic Bacteriology. Unfortunately, this scheme failed to describe many Cyanobacteria beyond the family level as indicated above. Similar results were achieved in previous studies [53, 37, 61]. A possible cause to the case in this study is that the spring temperature is evidently higher than the environmental temperature shifts caused by altitude, leading to a dominant influence on the bacterial community structure as indicated by the correlation analysis (Fig. 6).
Abundant unassigned-genus sequences were detected in this study, especially in LL-2 hot spring. That creates potential for existence of novel genera in these sites. Our recent culture-dependent study of cyanobacteria in these hot springs revealed existence of five phylotypes that were identified as putative new species related to Leptolyngbya or new genera [17]. Novel genera in these springs could be shaped by either of, or combination of two selective pressures: prolonged pressure of temperature (press), and extreme environmental perturbations (pulse) caused by frequent earthquakes. It remains to be seen how many of these lineages are novel and how will the communities change over time, but hot-springs of Ganzi remain one of interesting sites to study evolution of microbial populations shaped by extreme events for the years to come.
Conclusions
This is the first bacterial census of hot springs located in Western Sichuan, China, providing first insight into the microbial diversity in these geothermal fields. This study also highlighted the importance of temperature in sha** bacterial community structure of hot springs in Sichuan. Furthermore, abundant unassigned-genus sequences detected in this study strongly implied the presence of novel genera or genetic resources in these hot springs probably shaped by frequent tectonic activities.
Abbreviations
- MRPP:
-
Nonparametric multi-response permutation procedure
- NCBI:
-
National Centre for Biotechnology Information
- NMDS:
-
Non-metric multidimensional scaling
- OTU:
-
Operational taxonomic units
- PCoA:
-
Principal coordinate analysis
- RDP:
-
Ribosomal Database Project
- UPGMA:
-
Unweighted pair group method with arithmetic means
- YNP:
-
Yellowstone National Park
References
Inskeep WP, Jay ZJ, Tringe SG, Herrgård MJ, Rusch DB. The YNP metagenome project: environmental parameters responsible for microbial distribution in the Yellowstone geothermal ecosystem. Front Microbiol. 2013;4:67.
Everroad RC, Otaki H. Diversification of bacterial community composition along a temperature gradient at a thermal spring. Microbes Environ. 2012;27:374–81.
Cole JK, Peacock JP, Dodsworth JA, Williams AJ, Thompson DB, Dong H, et al. Sediment microbial communities in Great Boiling Spring are controlled by temperature and distinct from water communities. ISME J. 2013;7:718–29.
Reigstad LJ, Jorgensen SL, Schleper C. Diversity and abundance of Korarchaeota in terrestrial hot springs of Iceland and Kamchatka. ISME J. 2010;4:346–56.
Chia Sing C, Kok-Gan C, Yea-Ling T, Yi-Heng C, Kian Mau G. Diversity of thermophiles in a Malaysian hot spring determined using 16S rRNA and shotgun metagenome sequencing. Front Microbiol. 2015;6:177.
Hou W, Wang S, Dong H, Jiang H, Briggs BR, Peacock JP, et al. A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS One. 2013;8:e53350.
Jiang Z, Li P, Jiang D, Dai X, Zhang R, Wang Y, et al. Microbial community structure and arsenic biogeochemistry in an acid vapor-formed spring in Tengchong geothermal area, China. PLoS One. 2015;11:e0146331.
Wang S, Hou W, Dong H, Jiang H, Huang L, Wu G, et al. Control of temperature on microbial community structure in hot springs of the Tibetan Plateau. PLoS One. 2013;8:e62901.
Daroch M, Tang J, Jiang D, Luo Y, Liang Y, Li L, et al. Community structure and preliminary characterisation of thermophilic hot spring cyanobacteria of Western Sichuan, China. Phycologia. 2017;56:38–9.
Lin H. Subterranean hot water in Ganzi basin. Earthquake Research in Sichuan; 1983. p. 10–3.
Zhang J, Wuyang LI, Tang XC, Tian J, Wang YC, Guo Q, et al. Geothermal data analysis at the high-temperature hydrothermal area in Western Sichuan. Sci China Earth Sci. 2017;60:1–15.
Grant PR, Grant BR, Huey RB, Mtj J, Knoll AH, Schmitt J. Evolution caused by extreme events. Philos Trans R Soc Lond Ser B Biol Sci. 2017;372:20160146.
Lescak EA, Bassham SL, Catchen J, Gelmond O, Sherbick ML, von Hippel FA, et al. Evolution of stickleback in 50 years on earthquake-uplifted islands. P Natl Acad Sci USA. 2015;112:7204–12.
Jia Z, Liu Y, Daroch M, Geng S, Cheng JJ. Screening, growth medium optimisation and heterotrophic cultivation of microalgae for biodiesel production. Appl Biochem Biotechnol. 2014;173:1667–79.
Daroch M, Shao C, Liu Y, Geng S, Cheng JJ. Induction of lipids and resultant FAME profiles of microalgae from coastal waters of Pearl River Delta. Bioresour Technol. 2013;146:192–9.
Li JJ, Liu Y, Cheng JJ, Mos M, Daroch M. Biological potential of microalgae in China for biorefinery-based production of biofuels and high value compounds. New Biotechnol. 2015;32:588.
Tang J, Jiang D, Luo Y, Liang Y, Li L, Shah MMR, et al. Potential new genera of cyanobacterial strains isolated from thermal springs of western Sichuan, China. Algal Res. 2018;31:14–20.
Tringe SG, Hugenholtz P. A renaissance for the pioneering 16S rRNA gene. Curr Opin Microbiol. 2008;11:442–6.
Davies RJ-P, Craigie AI, Mackay DA, Whalen MA, Cheong JP-E, Leach GJ. Resolution of the taxonomy of Eriocaulon (Eriocaulaceae) taxa endemic to Australian mound springs, using morphometrics and AFLP markers. Aust Syst Bot. 2007;20:428–47.
Kikani B, Sharma A, Singh S. Culture dependent diversity and phylogeny of thermophilic Bacilli from a natural hot spring reservoir in the Gir Forest, Gujarat (India). Microbiology. 2015;84:687–700.
Ferris MJ, Muyzer G, Ward DM. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl Environ Microbiol. 1996;62:340–6.
Lepage E, Marguet E, Geslin C, Mattetailliez O, Zillig W, Forterre P, et al. Molecular diversity of new Thermococcales isolates from a single area of hydrothermal deep-sea vents as revealed by randomly amplified polymorphic DNA fingerprinting and 16S rRNA gene sequence analysis. Appl Environ Microbiol. 2004;70:1277–86.
Zhang H, Wang Y, Chen S, Zhao Z, Feng J, Zhang Z, et al. Water bacterial and fungal community compositions associated with urban lakes, **’an, China. Int J Environ Res Public Health. 2018;15:469.
Zhang H, Jia J, Chen S, Huang T, Wang Y, Zhao Z, et al. Dynamics of bacterial and fungal communities during the outbreak and decline of an algal bloom in a drinking water reservoir. Int J Environ Res Public Health. 2018;15:361.
Zhang H, Feng J, Chen S, Li B, Sekar R, Zhao Z, et al. Disentangling the drivers of diversity and distribution of fungal community composition in wastewater treatment plants across spatial scales. Front Microbiol. 2018;9:1291. https://doi.org/10.3389/fmicb.2018.0129.
Quince C, Lanzén A, Curtis TP, Davenport RJ, Hall N, Head IM, et al. Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Methods. 2009;6:639–41.
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–20.
Caporaso JG, Lauber CL, Walters WA, Berglyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4.
Avijeet Singh O, Oinam G, Ojit Singh K, Tiwari ON. Isolation of fresh water Cyanobacterial DNA of north east India by modified Xanthogenate method. Int J Res BioSciences;2013;2(2):75–82.
Wu Y, Tan L, Liu W, Wang B, Wang J, Cai Y, et al. Profiling bacterial diversity in a limestone cave of the western Loess Plateau of China. Front Microbiol. 2014;6:244.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–63.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8.
Guang-Hai FU, Yin JC. A study on the distribution and general mechanism about the hot spring as well as tourism development in Ganzi of Sichuan Province. J Northwest Univ. 2009;39:142–8.
Purcell D, Sompong U, Yim LC, Barraclough TG, Peerapornpisal Y, Pointing SB. The effects of temperature, pH and sulphide on the community structure of hyperthermophilic streamers in hot springs of northern Thailand. FEMS Microbiol Ecol. 2007;60:456–66.
Huang Q, Dong CZ, Dong RM, Jiang H, Wang S, Wang G, et al. Archaeal and bacterial diversity in hot springs on the Tibetan Plateau, China. Extremophiles. 2011;15:549–63.
Ghilamicaelet MA, Budambula NLM, Anami SE, Mehari T, Boga HI. Evaluation of prokaryotic diversity of five hot springs in Eritrea. BMC Microbiology. 2017;17:203. https://doi.org/10.1186/s12866-017-1113-4.
Pedersen D, Miller SR. Photosynthetic temperature adaptation during niche diversification of the thermophilic cyanobacterium Synechococcus A/B clade. ISME J. 2016;11:1053–7.
Chiriac CM, Szekeres E, Rudi K, Baricz A, Hegedus A, Dragoş N, et al. Differences in temperature and water chemistry shape distinct diversity patterns in thermophilic microbial communities. Appl Environ Microbiol. 2017;83:e01363–17.
López-López O, Knapik K, Cerdán ME, González-Siso MI. Metagenomics of an alkaline hot spring in Galicia (Spain): microbial diversity analysis and screening for novel lipolytic enzymes. Front Microbiol. 2015;6.
Iva T, Margarita SD, Dimitrina L, Javier P, Peter P, Margarita K. Phylogenetic analysis of the bacterial community in a geothermal spring, Rupi Basin, Bulgaria. World J Microbiol Biotechnol. 2010;26:2019–28.
Inskeep WP, Rusch DB, Jay ZJ, Herrgard MJ, Kozubal MA, Richardson TH, et al. Metagenomes from high-temperature chemotrophic systems reveal geochemical controls on microbial community structure and function. PLoS One. 2010;5:e9773.
León KBD, Gerlach R, Peyton BM, Fields MW. Archaeal and bacterial communities in three alkaline hot springs in Heart Lake Geyser Basin, Yellowstone National Park. Front Microbiol. 2013;4:330.
Briggs BR, Brodie EL, Tom LM, Dong H, Jiang H, Huang Q, et al. Seasonal patterns in microbial communities inhabiting the hot springs of Tengchong, Yunnan Province, China. Environ Microbiol. 2014;16:1579–91.
Yim LC, Hongmei J, Aitchison JC, Pointing SB. Highly diverse community structure in a remote central Tibetan geothermal spring does not display monotonic variation to thermal stress. FEMS Microbiol Ecol. 2006;57:80–91.
Tyson GW, Chapman J, Hugenholtz P, Allen EE, Ram RJ, Richardson PM, et al. Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature. 2004;428:37–43.
Whitton B. Ecology of cyanobacteria II: their diversity in space and time. 1st edition, Springer; 2012. ISBN 978-94-007-3854-6. https://doi.org/10.1007/978-94-007-3855-3.
Costa KC, Navarro JB, Shock EL, Zhang CL, Soukup D, Hedlund BP. Microbiology and geochemistry of great boiling and mud hot springs in the United States Great Basin. Extremophiles. 2009;13:447–59.
Meyerdombard DR, Swingley W, Raymond J, Havig J, Shock EL, Summons RE. Hydrothermal ecotones and streamer biofilm communities in the Lower Geyser Basin, Yellowstone National Park. Environ Microbiol. 2011;13:2216–31.
Reysenbach AL, Banta A, Civello S, Daly J, Mitchel K, Lalonde S, et al. Aquificales in Yellowstone National Park. In: McDermott TR, Inskeep WP, editors. Geothermal Biology and Geochemistry in YNP. Bozeman: Montana State University; 2005. p. 129–42.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7.
Bolhuis H, Stal LJ. Analysis of bacterial and archaeal diversity in coastal microbial mats using massive parallel 16S rRNA gene tag sequencing. ISME J. 2011;5:1701–12.
**g H, **a X, Suzuki K, Liu H. Vertical profiles of bacteria in the tropical and subarctic oceans revealed by pyrosequencing. PLoS One. 2013;8:e79423.
Komárek J. Recent changes (2008) in cyanobacteria taxonomy based on a combination of molecular background with phenotype and ecological consequences (genus and species concept). Hydrobiologia. 2010;639:245–59.
Panda AK, Bisht SS, De MS, Kumar NS. Bacterial and archeal community composition in hot springs from Indo-Burma region, North-east India. AMB Express. 2016;6:111.
Amin A, Ahmed I, Salam N, Kim BY, Singh D, Zhi XY, et al. Diversity and distribution of thermophilic Bacteria in hot springs of Pakistan. Microb Ecol. 2017;74:1–12.
Chan CS, Chan KG, Ee R, Hong KW, Urbieta MS, Donati ER, et al. Effects of physiochemical factors on prokaryotic biodiversity in Malaysian Circumneutral Hot Springs. Front Microbiol. 2017;8:244.
Blank CE, Cady SL, Pace NR. Microbial composition of near-boiling silica-depositing thermal springs throughout Yellowstone National Park. Appl Environ Microbiol. 2002;68:5123–35.
Sanka LD, Sadaiappan B, Poosakkannu A, Muthuraman S. Pyrosequencing-based seasonal observation of prokaryotic diversity in pneumatophore-associated soil of Avicennia marina. Curr Microbiol. 2016;72:1–7.
Singh Y, Gulati A, Singh DP, Khattar JIS. Cyanobacterial community structure in hot water springs of Indian North-Western Himalayas: a morphological, molecular and ecological approach. Algal Res. 2018;29:179–92.
Acknowledgments
Authors would like to thank Professor Li ** of Sichuan Agricultural University for access to the laboratory for initial sample preservation and DNA isolation.
Funding
This work was supported by Start-up Fund to Jie Tang from Chengdu University, China [2081917012]; National Natural Science Foundation of China for Young International Scientists [31550110497]; and Shenzhen Knowledge and Innovation Basic Research Grant [JCYJ20160122151433832], both to Maurycy Daroch. Funding bodies had no influence on the way study was conducted.
Availability of data and materials
The data is available on NCBI Sequence Read Archive with accession numbers SRR5104369-5104384. Hot springs: Sichuan, China.
Author information
Authors and Affiliations
Contributions
MD and JT conceived and designed research. MD, MMRS, LHL and YL conducted sampling. DJ, YML and MD performed experimental procedures. JT, YFL and MD performed analyses and interpreted data. All authors participated in preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Recent earthquakes in Ganzi prefecture. (DOCX 36 kb)
Additional file 2:
Table S2. Statistical summary of sequencing by MiSeq platform. (DOCX 35 kb)
Additional file 3:
Figure S1. Rarefaction curves. (DOCX 106 kb)
Additional file 4:
Table S3. The relative abundances (%) at phylum level in different samples. (DOCX 42 kb)
Additional file 5:
Table S4. The relative abundances (%) at genus level in different sample (DOCX 44 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tang, J., Liang, Y., Jiang, D. et al. Temperature-controlled thermophilic bacterial communities in hot springs of western Sichuan, China. BMC Microbiol 18, 134 (2018). https://doi.org/10.1186/s12866-018-1271-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-018-1271-z