Abstract
Autoimmune diseases are often characterized as clinical syndromes caused by the inappropriate activation of T or B cells resulting in systemic or organ-specific damage. However, studies support a role for the innate immune system, and in particular natural killer (NK) cells, in stimulating or suppressing autoimmunity. This review focuses on recent research elucidating a potential immunoregulatory role for NK cells in modulating T and B cell-mediated autoimmunity.
Similar content being viewed by others
Natural killer cells
Natural killer (NK) cells are large, granular, bone marrow-derived lymphocytes that do not express T or B cell receptors [1]. In humans, these CD3-negative cells are identified by the surface markers CD16 and CD56 and comprise 5–15% of the peripheral blood mononuclear cells in normal individuals [2]. Although they were initially identified by their ability to lyse tumor cells without prior sensitization, more recent work has demonstrated that they have a crucial role in the initial defense against pathogens and are particularly important in responding to viral infections (reviewed in [3, 4]).
NK cells are poised for a rapid response to infected or transformed cells either by killing the abnormal cells or by releasing immunomodulatory chemokines and cytokines such as interferon-γ (IFN-γ). The chemokines and cytokines released by NK cells influence the initiation and development of the subsequent adaptive immune response [5–9]. Clearly, NK cell functions must be carefully regulated to prevent damage to normal tissues or the indiscriminate release of cytokines resulting in inappropriate activation of the adaptive immune system.
NK cell responses result from the integration of signals from both cytokine receptors and germline-encoded NK cell inhibitory and activation receptors. NK cell receptors include the murine C-type lectin-like receptors in the Ly49 family, the C-type lectin-like receptors shared by mouse and human (for example NKR-P1, NKG2D and CD94/NKG2A), the human immunoglobulin-like receptors in the killer immunoglobulin-like receptor (KIR) family, and the immunoglobulin-like receptors shared by both mouse and human (for example 2B4) including the natural cytotoxicity receptor family (Nkp30, Nkp44 and Nkp46; reviewed in [1, 10–13]).
Although NK cells are prepared to kill abnormal cells and rapidly release cytokines, they are normally restrained by inhibitory receptors that recognize target-cell-expressed MHC class I molecules and allow NK cells to survey tissues for normal MHC class I expression (Fig. 1). When MHC class I molecules are downregulated or absent, NK cells are released from the inhibitory influence of these receptors and kill target cells more efficiently ('missing self' hypothesis) [14]. It has been proposed that NK cells all express at least one inhibitory receptor that recognizes self MHC to provide NK cell tolerance and to prevent inappropriate NK cell responses directed at self [15, 16]. However, release from inhibitory receptor effects does not automatically lead to NK cell activation against cellular targets. NK cells also express different combinations of various activation receptors, allowing them to respond to ligands on potential target cells [17–20]. The ligands for activation receptors are often closely related to the ligands for inhibitory receptors. In other cases, the activation receptor ligands may be upregulated in response to stress or infection, as illustrated by the upregulation of the NKG2D ligand MHC class I-related chain A (MICA) during infection [21–23].
Natural killer (NK) cell activation is controlled by the integration of signals from activation and inhibitory receptors. (a) Inhibitory NK cell receptors recognize self MHC class I and restrain NK cell activation. (b) When unimpeded by the inhibitory receptors, binding of NK cell activation receptors to their ligands on target cells results in NK cell stimulation. In the absence or downregulation of self MHC class I on the target cells, these stimulatory signals are no longer suppressed, resulting in NK cell responses including cytokine production and granule release leading to cytotoxicity. Note that this model indicates that NK cells do not kill by default; that is, when MHC class I inhibition is absent, the NK cell must still be stimulated through activation receptors. Moreover, whether or not an individual NK cell is activated by a target is determined by this complex balance of receptors with opposing function and expression of the corresponding ligands. In general, however, inhibition dominates over activation. Finally, NK cells can be directly stimulated by cytokines such as interleukin-12 that trigger the production of other cytokines by NK cells (not shown). These direct cytokine-mediated responses are not affected by MHC class I expression.
NK cells can also respond directly to cytokines such as interleukin (IL)-12 and IL-18 that stimulate their production of other cytokines, including IFN-γ (not shown in Fig. 1). Such cytokine-mediated responses are generally not regulated by MHC class I expression, although MHC class I expression does regulate target-cell-stimulated cytokine release (Fig. 1).
Observations of NK cell abnormalities in human autoimmune diseases
Since the early 1980s, many studies have documented decreased NK cell numbers and impairment of NK cell function in the peripheral blood of patients with autoimmune diseases such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), Sjögren's syndrome, rheumatoid arthritis (RA), and type I diabetes (reviewed in [24–26]). However, some of the older reports did not distinguish between NK cells and NKT cells, a potential immunoregulatory subpopulation of T cells that are also able to rapidly release large amounts of cytokines. Better discrimination between these two populations has shown that in addition to the decreased NK cell numbers found in many autoimmune diseases, NKT cells are decreased in RA and psoriasis [27, 28], but this topic is beyond the scope of this review.
More recent reports have also shown an association between NK cell deficits and autoimmune thyroid disease [29, 30] and psoriasis [31] as well as several pediatric rheumatologic diseases including juvenile dermatomyositis [32] or SLE [33]. Low NK cell numbers have also been found in patients with systemic-onset juvenile rheumatoid arthritis (JRA) [34], and decreased NK cell function has been documented in systemic-onset JRA patients with macrophage activation syndrome or hemophagocytic lymphohistiocytosis [35–37].
Although there is substantial evidence correlating decreased NK cell numbers and function with autoimmune diseases, it is not clear whether the reported NK cell alterations occur secondarily to the disease and its treatments or are primary defects involved in the disease pathogenesis. However, recent studies have documented NK cell defects in treatment-naïve patients before overt progression to disease or at the time of diagnosis, demonstrating that the defects are not solely treatment-related or the result of chronic inflammation from long-standing disease [32, 33]. In addition, a temporal correlation has been identified between NK cell numbers or activity and periods of disease progression or remission in MS and SLE, suggesting that NK cells may play an immunoregulatory role in disease pathology [38–43].
The correlation of decreased NK cell numbers and/or function with autoimmune diseases raises the possibility that autoimmunity may arise from NK cell deficiencies. However, such conclusions must be tempered by the fact that reports of NK cell defects in patients with autoimmune diseases have been based almost exclusively on studies of peripheral blood samples, which would not distinguish between true deficits and sequestration of NK cells in target tissues. In addition, the clinical course of patients with selective, complete NK cell deficiencies has been dominated by overwhelming infections, often from herpesviruses, and not by autoimmune syndromes [44–46]. In contrast, NK cell lymphocytosis and leukemia are associated with autoimmune syndromes, such as vasculitis and RA [47–49], suggesting that peripheral-blood NK cell defects in systemic autoimmune syndromes may be due to the sequestration of NK cells in target tissues.
Alterations in KIR expression on NK cells (and/or on T cells) have also been associated with autoimmune diseases such as Behcet's disease, type I diabetes, and psoriasis [50–52]. In addition, aberrant expression of NK cell receptors on subpopulations of CD4+ T cells has been observed in patients with RA [53–56]. Skewed KIR receptor repertoires may potentially lower the threshold for NK cell or T cell activation when activating forms of the immunoglobulin-like receptors (KARs) are present either in the absence of their corresponding inhibitory KIRs or in the absence of the HLA ligands for the inhibitory KIRs [50–53, 55].
Abnormal expression of another NK cell activation receptor, NKG2D, has also been observed on a fraction of CD4+ T cells in patients with RA [57]. Furthermore, expression of MICA, an inducible ligand for the NKG2D [58], has also been identified in the synovium of patients with RA, suggesting that the abnormal expression of MICA in the context of upregulation of NKG2D on CD4+ T cells in patients with RA may result in this subpopulation of CD4+ T cells receiving persistent co-stimulation [57]. In addition to potentially prolonging T-cell activation, the aberrant expression of MICA may also be involved in modulating the responses of NK cells in the inflamed synovium.
Indeed, NK cells comprise a significant fraction of the lymphocytes found in the synovial fluid of patients with RA (8–16% of lymphocytes) and can be detected in the joint at an early stage in the disease course [59]. Interestingly, most of the NK cells in the synovial fluid of patients with established RA are CD56bright, with elevated expression of both CD94 and NKG2A and decreased expression of KIRs and CD16 [60, 61]. The CD56bright subpopulation of NK cells is also found in the blood of patients with RA and normal controls but at much lower frequencies (typically 10% of NK cells). Although originally thought to be immature NK cells, evidence now supports the hypothesis that CD56bright NK cells are a separate subpopulation that produce high levels of cytokines and may potentially play a role in immunoregulation [62, 63]. The NK cells within the synovium also have an upregulated expression of several chemokine receptors and adhesion molecules that may participate in preferential recruitment into the synovium [60]. It is proposed that the unusual enrichment of CD56bright NK cells may have a role in the initiation and/or perpetuation of dysregulated production of proinflammatory cytokines in the synovium of patients with RA [60, 61]. It is also possible that aberrant expression of MICA in the inflamed synovium [57] is involved in modulating the responses of this subset of NK cells, resulting in the dysregulated production of proinflammatory cytokines rather than in immunoregulation.
Most of the evidence from human autoimmune diseases suggests that NK cells may be involved in immunoregulation and/or autoimmune disease pathogenesis. However, because it is difficult to determine potential mechanistic roles of NK cells in these diseases and to rule out epiphenomena in humans, we will now shift our focus to mouse models of autoimmunity.
Insight into the role of NK cells in autoimmunity from mouse models of autoimmune diseases
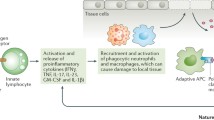
The role of NK cells in autoimmune responses has been examined in several murine models of autoimmune diseases (reviewed in [25, 26]). The evidence from these studies (discussed below) suggests that NK cells can affect the development of autoimmunity through several mechanisms, including suppressing viral infections and potential subsequent autoimmune responses, modulating autoreactive responses of other immune cells, or, as effector cells, directly mediating tissue damage (Fig. 2). Different NK cell responses in these models presumably result from alterations in the balance between inhibitory and stimulatory signals mediated through the interactions of NK cell receptors and their ligands. The expression levels of ligands for both inhibitory and activation NK cell receptors in target tissues as well as the immediate cytokine milieu modulate the NK cell activation threshold, allowing different NK cell responses that could potentially suppress or augment autoimmunity.
Murine models show that natural killer (NK) cells affect autoimmunity through several potential mechanisms. (a) NK cells limit viral-induced tissue damage by directly killing virally infected cells or by releasing cytokines that can suppress viral propagation either directly or indirectly by activating other cells such as macrophages. Defective NK cell responses to viral infections may result in autoimmunity in genetically predisposed strains of mice as a result of uncontrolled infection leading to increased tissue destruction, with accompanying exposure of self antigens. (b) NK cells participate in the immunoregulation of other immune cells. Control of autoreactive T and B cells by NK cells may be mediated directly through the release of cytokines and chemokines or indirectly through bidirectional interactions with other components of the innate immune system such as dendritic cells (DCs). In addition, it is possible that NK cells may kill autoreactive lymphocytes or inappropriately activated immature DCs. (c) NK cells could potentially mediate an autoimmune response by inappropriately killing normal tissues.
Viral infections have been implicated in the pathogenesis of several autoimmune diseases due to molecular mimicry or polyclonal immune activation [64]. It is well established that NK cells have a crucial role in the initial defense against viral infections [3, 4]. It is therefore not surprising that several investigators have attributed the impact of relative deficiencies of NK cell numbers or function seen in many autoimmune diseases to a decreased ability to respond to viral infections.
Results from mouse models show a role for NK cells in suppressing autoimmune responses after viral infections (Fig. 2a). For example, NK cells are important in preventing encephalitis in a murine model of MS induced by Theiler's murine encephalitis virus [65]. Depletion of NK cells in resistant mice resulted in the development of diffuse encephalitis and meningitis early in the post-infection period [65]. NK cells are also thought to prevent coxsackievirus B3 (CVB3)-induced myocarditis by limiting viral replication and thus preventing prolonged immune activation and minimizing tissue damage with exposure of self antigens. Infection with either CVB3 or murine cytomegalovirus resulted in chronic myocarditis in susceptible strains of mice, accompanied by the development of autoantibodies against cardiac myosin [66]. Depletion of NK cells before infection with CVB3 rendered resistant strains of mice as sensitive to the development of myocarditis as susceptible strains of mice [66]. Thus, NK cells may have a role in suppressing autoimmunity after viral infections by effectively limiting viral replication and subsequent tissue destruction.
Evidence from murine models also supports an immunoregulatory role for NK cells in modulating other immune cell responses (Fig. 2b). The development of autoimmunity in C57BL/6lpr mice, which have a defect in Fas, a gene encoding a tumor necrosis factor receptor superfamily member involved in inducing apoptosis, is temporally related to an age-dependent loss of NK and NKT cells. Furthermore, antibody-mediated NK cell depletion in these mice enhanced the development of autoantibody-secreting B cells, whereas the adoptive transfer of NK cells delayed the onset of autoantibody production [67]. Studies in vitro have shown that rat NK cells can inhibit autoreactive T cell cytokine production and proliferation [68]. Indeed, depletion of NK cells worsened colitis in a CD4+ T cell transfer model in mice, demonstrating an immunoregulatory role for NK cells [69]. Several investigators have reported similar findings in experimental autoimmune encephalomyelitis (EAE), a Th1-mediated mouse model of MS [70, 71]. Depletion of NK cells before immunization of sensitive mice with myelin oligodendrocyte glycoprotein (MOG35–55) peptide resulted in clinically more severe, relapsing EAE [70]. Depletion of NK cells also resulted in more severe disease after passive transfer of an EAE-inducing CD4+ T cell line, showing that NK cells are not only involved in the initiation of EAE but can inhibit effector T cells [70]. NK cell depletion in rats before immunization with myelin basic protein also exacerbated the clinical features of EAE and increased mortality [71]. However, these results conflict with a third study, which reported that NK cell depletion resulted in less severe clinical scores [72]. Overall, NK cells appear to participate in regulating T and B cell-mediated autoimmune responses.
Less information is available on how NK cells perform this potential immunoregulatory role. It is possible that this role is mediated directly by the release of immunomodulatory cytokines and chemokines involved in lymphocyte recruitment, activation, and suppression, such as IFN-γ and transforming growth factor-β. However, NK cell-derived cytokines and chemokines may act indirectly by inducing cytokine production in other cells, activating macrophages, or supporting the maturation of dendritic cells (DCs). Indeed, bidirectional interactions between NK cells and other components of the innate immune system such as DCs and NKT cells have been reported ([73–75]; reviewed in [9, 76]). It is also possible that the immunoregulatory role of NK cells is mediated by the killing of autoreactive lymphocytes or immature DCs by NK cells. The immunoregulatory role of NK cells in suppressing colitis in a murine CD4+ T cell transfer model was found to be dependent on perforin, suggesting that the NK cells were directly killing autoreactive T cells or some other intermediate effector cells such as DCs [69]. In addition, several studies have shown that NK cells are potentially able to influence the subsequent adaptive immune response by lysing immature DCs [74, 75] or develo** T cells [77]. Therefore, it appears that NK cells may employ several different mechanisms to regulate the responses of other immune cells and thereby affect the development of autoimmunity.
Murine models of other autoimmune diseases suggest that NK cells may also participate in the initiation of autoimmunity through interactions with autoreactive T and B cells. Experimental autoimmune myasthenia gravis (EAMG) is an antibody-mediated autoimmune disease in which autoantibodies against the acetylcholine receptor (AchR) in neuromuscular junctions are stimulated in susceptible mice by repeated immunizations with Torpedo AChR in adjuvant. Depletion of NK cells before immunization resulted in significantly delayed onset and decreased severity of EAMG with decreased anti-AChR antibody production [78]. Interestingly, NK cell depletion after the initial immunization had no impact on the development of EAMG. In a mouse model of asthma, initial immunization with ovalbumin in adjuvant followed by repeated daily exposure to aerosolized ovalbumin resulted in CD4+ T cell-dependent pulmonary eosinophilic inflammation and systemic IgE production, consistent with a Th2 immune response [79]. Depletion of NK cells before the initial immunization but not later during the challenge period resulted in a diminished infiltration of pulmonary eosinophils and CD3+ T cells as well as a decreased systemic production of IgE, suggesting a role for NK cells in promoting allergen-induced airway inflammation [79]. Interestingly, the temporal impact of NK depletion observed in these models has also been reported in an EAE model in which the depletion of NK cells after the primary immunization did not impact development of EAE [72]. These results suggest that NK cells may be most influential at the initiation of the autoimmune response.
In addition to potential immunoregulatory roles for NK cells in autoimmunity, NK cell-mediated cytotoxicity may result directly in significant organ-specific damage (Fig. 2c). Several groups have shown that activated NK cells can lyse autologous neurons in vitro, suggesting that NK cell cytotoxity may have a role in EAE [80, 81]. NK cells have also been implicated in the selective neuronal death in the superior cervical ganglia of rats treated with guanethidine [82]. Recent experiments have shown that NK cells can kill syngeneic dorsal root ganglia neurons by a perforin-dependent mechanism [83]. Interestingly, it was shown that this response was mediated by NKG2D recognition of a ligand on dorsal root ganglia neurons that was not expressed on resistant central nervous system-derived neurons [83]. NK cell-mediated killing of syngeneic neurons expressing an NK cell activation receptor ligand supports the hypothesis that inappropriate killing of self tissues by NK cells may reflect a loss of NK cell 'tolerance' occurring in tissues that have inappropriately downregulated MHC class I ligands for NK cell inhibitory receptors or that have aberrant expression of ligands for NK cell activation receptors or expression of these ligands in tissues that are normally isolated from NK cells.
NK cells appear to participate in mediating organ-specific damage in several murine models of autoimmunity. Experimental autoimmune uveoretinitis (EAU) is induced by immunizing sensitive strains of mice with ocular autoantigens. Depletion of NK cells before immunization resulted in significantly less severe EAU, demonstrating that NK cells participate in the development of EAU, either by directly mediating cellular damage or by supporting rather than suppressing autoreactive T cells [84]. A murine model of autoimmune-mediated diabetes after viral infection with CVB4 provides another example of organ-specific, NK cell-mediated damage [85]. In this model, mice whose pancreatic beta cells express a transgene for the suppressor of cytokine signaling (SOCS-1), an inhibitor of interferon signaling, develop diabetes soon after CVB4 infection. However, depletion of NK cells before infection with CVB4 prevented the development of diabetes, implying that NK cells contributed to the destruction of the infected pancreatic beta cells, although no direct evidence was presented to show the involvement of NK cell-mediated cytotoxicity [85]. Therefore, in spite of in vitro data suggesting that NK cell-mediated cytotoxicity may result in organ-specific autoimmunity, more direct in vivo experimental evidence in murine models is needed to support this hypothesis.
Conclusions
There is strong evidence that the innate immune system, and in particular NK cells, influence subsequent adaptive immune responses. By virtue of their ability to rapidly kill abnormal cells and produce cytokines and chemokines, NK cells are positioned for a key role in regulating autoimmune responses. The results summarized in this review demonstrate that NK cells are involved in modulating responses to self antigens and that in some circumstances NK cells can either suppress or augment autoimmunity, directly or indirectly. The associations found in humans and the empirical evidence from murine models suggest that further research into the immunmodulatory role of NK cells in autoimmunity is warranted and is likely to provide new insights into the pathogenesis of autoimmune disorders.
Abbreviations
- AchR:
-
= acetylcholine receptor
- CV:
-
= coxsackievirus
- DC:
-
= dendritic cell
- EAE:
-
= experimental autoimmune encephalomyelitis
- EAMG:
-
= experimental autoimmune myasthenia gravis
- EAU:
-
= experimental autoimmune uveoretinitis
- IFN-γ:
-
= interferon-γ
- IL:
-
= interleukin
- JRA:
-
= juvenile rheumatoid arthritis
- KIR:
-
= killer immunoglobulin-like receptor
- MHC:
-
= major histocompatibility complex
- MICA:
-
= MHC class I-related chain A
- MS:
-
= multiple sclerosis
- NK:
-
= natural killer
- RA:
-
= rheumatoid arthritis
- SLE:
-
= systemic lupus erythematosus
- Th:
-
= T helper cells.
References
Yokoyama WM: Natural killer cell receptors. Curr Opin Immunol. 1998, 10: 298-305. 10.1016/S0952-7915(98)80168-4.
Seaman WE: Natural killer cells and natural killer T cells. Arthritis Rheum. 2000, 43: 1204-1217. 10.1002/1529-0131(200006)43:6<1204::AID-ANR3>3.0.CO;2-I.
Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP: Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999, 17: 189-220. 10.1146/annurev.immunol.17.1.189.
French AR, Yokoyama WM: Natural killer cells and viral infections. Curr Opin Immunol. 2003, 15: 45-51. 10.1016/S095279150200002X.
Horwitz DA, Gray JD, Ohtsuka K, Hirokawa M, Takahashi T: The immunoregulatory effects of NK cells – the role of TGF-β and implications for autoimmunity. Immunol Today. 1997, 18: 538-542. 10.1016/S0167-5699(97)01149-3.
Fearon DT, Locksley RM: The instructive role of innate immunity in the acquired immune response. Science. 1996, 272: 50-53.
Kos FJ: Regulation of adaptive immunity by natural killer cells. Immunol Res. 1998, 17: 303-312.
Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA: NK cell functions restrain T cell responses during viral infections. Eur J Immunol. 2001, 31: 3048-3055. 10.1002/1521-4141(2001010)31:10<3048::AID-IMMU3048>3.0.CO;2-1.
Zitvogel L: Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J Exp Med. 2002, 195: F9-F14. 10.1084/jem.20012040.
Lanier LL: NK cell receptors. Annu Rev Immunol. 1998, 16: 359-393. 10.1146/annurev.immunol.16.1.359.
Campbell KS, Colonna M: Human natural killer cell receptors and signal transduction. Int Rev Immunol. 2001, 20: 333-370.
Moretta L, Biassoni R, Bottino C, Cantoni C, Pende D, Mingari MC, Moretta A: Human NK cells and their receptors. Microbes Infect. 2002, 4: 1539-1544. 10.1016/S1286-4579(02)00037-0.
Yokoyama WM, Plougastel BF: Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003, 3: 304-316. 10.1038/nri1055.
Ljunggren HG, Karre K: In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990, 11: 237-244. 10.1016/0167-5699(90)90097-S.
Raulet DH: Development and tolerance of natural killer cells. Curr Opin Immunol. 1999, 11: 129-134. 10.1016/S0952-7915(99)80023-5.
Raulet DH, Vance RE, McMahon CW: Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001, 19: 291-330. 10.1146/annurev.immunol.19.1.291.
Lanier LL: On guard – activating NK cell receptors. Nat Immunol. 2001, 2: 23-27. 10.1038/83130.
Diefenbach A, Raulet DH: Strategies for target cell recognition by natural killer cells. Immunol Rev. 2001, 181: 170-184. 10.1034/j.1600-065X.2001.1810114.x.
Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L: Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001, 19: 197-223. 10.1146/annurev.immunol.19.1.197.
Smith HR, Idris AH, Yokoyama WM: Murine natural killer cell activation receptors. Immunol Rev. 2001, 181: 115-125. 10.1034/j.1600-065X.2001.1810109.x.
Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T: Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001, 2: 255-260. 10.1038/85321.
Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF: MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity. 2001, 15: 83-93. 10.1016/S1074-7613(01)00168-6.
Tieng V, Le Bouguenec C, du Merle L, Bertheau P, Desreumaux P, Janin A, Charron D, Toubert A: Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc Natl Acad Sci USA. 2002, 99: 2977-2982. 10.1073/pnas.032668099.
Grunebaum E, Malatzky-Goshen E, Shoenfeld Y: Natural killer cells and autoimmunity. Immunol Res. 1989, 8: 292-304.
Flodstrom M, Shi FD, Sarvetnick N, Ljunggren HG: The natural killer cell – friend or foe in autoimmune disease?. Scand J Immunol. 2002, 55: 432-441. 10.1046/j.1365-3083.2002.01084.x.
Baxter AG, Smyth MJ: The role of NK cells in autoimmune disease. Autoimmunity. 2002, 35: 1-14. 10.1080/08916930290005864.
Yanagihara Y, Shiozawa K, Takai M, Kyogoku M, Shiozawa S: Natural killer (NK) T cells are significantly decreased in the peripheral blood of patients with rheumatoid arthritis (RA). Clin Exp Immunol. 1999, 118: 131-136. 10.1046/j.1365-2249.1999.01018.x.
Koreck A, Suranyi A, Szony BJ, Farkas A, Bata-Csorgo Z, Kemeny L, Dobozy A: CD3+CD56+ NK T cells are significantly decreased in the peripheral blood of patients with psoriasis. Clin Exp Immunol. 2002, 127: 176-182. 10.1046/j.1365-2249.2002.01721.x.
Bossowski A, Urban M, Stasiak-Barmuta A: Analysis of circulating T gamma/delta lymphocytes and CD16/56 cell populations in children and adolescents with Graves' disease. Pediatr Res. 2003, 54: 425-429. 10.1203/01.PDR.0000076663.94850.44.
Ciampolillo A, Guastamacchia E, Amati L, Magrone T, Munno I, Jirillo E, Triggiani V, Fallacara R, Tafaro E: Modifications of the immune responsiveness in patients with autoimmune thyroiditis: evidence for a systemic immune alteration. Curr Pharm Des. 2003, 9: 1946-1950. 10.2174/1381612033454270.
Cameron AL, Kirby B, Griffiths CE: Circulating natural killer cells in psoriasis. Br J Dermatol. 2003, 149: 160-164. 10.1046/j.1365-2133.2003.05319.x.
O'Gorman M, Smith R, Garrison A, Shamiyeh E, Pachman L: Lymphocyte subsets in peripheral blood from newly diagnosed, untreated patients with juvenile dermatomyositis (JDM) are associated with disease activity scores (DAS). Arthritis Rheum. 2002, 46 (suppl 9): S490-
Yabuhara A, Yang FC, Nakazawa T, Iwasaki Y, Mori T, Koike K, Kawai H, Komiyama A: A killing defect of natural killer cells as an underlying immunologic abnormality in childhood systemic lupus erythematosus. J Rheumatol. 1996, 23: 171-177.
Wouters CH, Ceuppens JL, Stevens EA: Different circulating lymphocyte profiles in patients with different subtypes of juvenile idiopathic arthritis. Clin Exp Rheumatol. 2002, 20: 239-248.
Wulffraat NM, Rijkers GT, Elst E, Brooimans R, Kuis W: Reduced perforin expression in systemic juvenile idiopathic arthritis is restored by autologous stem-cell transplantation. Rheumatology (Oxford). 2003, 42: 375-379. 10.1093/rheumatology/keg074.
Imashuku S, Hyakuna N, Funabiki T, Ikuta K, Sako M, Iwai A, Fukushima T, Kataoka S, Yabe M, Muramatsu K, Kohdera U, Nakadate H, Kitazawa K, Toyoda Y, Ishii E: Low natural killer activity and central nervous system disease as a high-risk prognostic indicator in young patients with hemophagocytic lymphohistiocytosis. Cancer. 2002, 94: 3023-3031. 10.1002/cncr.10515.
Grom AA, Villanueva J, Lee S, Goldmuntz EA, Passo MH, Filipovich A: Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr. 2003, 142: 292-296. 10.1067/mpd.2003.110.
Erkeller-Yusel F, Hulstaart F, Hannet I, Isenberg D, Lydyard P: Lymphocyte subsets in a large cohort of patients with systemic lupus erythematosus. Lupus. 1993, 2: 227-231.
Erkeller-Yuksel FM, Lydyard PM, Isenberg DA: Lack of NK cells in lupus patients with renal involvement. Lupus. 1997, 6: 708-712.
Munschauer FE, Hartrich LA, Stewart CC, Jacobs L: Circulating natural killer cells but not cytotoxic T lymphocytes are reduced in patients with active relapsing multiple sclerosis and little clinical disability as compared to controls. J Neuroimmunol. 1995, 62: 177-181. 10.1016/0165-5728(95)00115-9.
Kastrukoff LF, Morgan NG, Zecchini D, White R, Petkau AJ, Satoh J, Paty DW: A role for natural killer cells in the immunopathogenesis of multiple sclerosis. J Neuroimmunol. 1998, 86: 123-133. 10.1016/S0165-5728(98)00014-9.
Riccieri V, Spadaro A, Parisi G, Taccari E, Moretti T, Bernardini G, Favaroni M, Strom R: Down-regulation of natural killer cells and of gamma/delta T cells in systemic lupus erythematosus. Does it correlate to autoimmunity and to laboratory indices of disease activity?. Lupus. 2000, 9: 333-337. 10.1191/096120300678828460.
Takahashi K, Miyake S, Kondo T, Terao K, Hatakenaka M, Hashimoto S, Yamamura T: Natural killer type 2 bias in remission of multiple sclerosis. J Clin Invest. 2001, 107: R23-R29.
Biron CA, Byron KS, Sullivan JL: Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989, 320: 1731-1735.
Jawahar S, Moody C, Chan M, Finberg R, Geha R, Chatila T: Natural Killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II). Clin Exp Immunol. 1996, 103: 408-413.
Orange JS: Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002, 4: 1545-1558. 10.1016/S1286-4579(02)00038-2.
Lamy T, Loughran TP: Clinical features of large granular lymphocyte leukemia. Semin Hematol. 2003, 40: 185-195. 10.1016/S0037-1963(03)00133-1.
Tefferi A, Li CY, Witzig TE, Dhodapkar MV, Okuno SH, Phyliky RL: Chronic natural killer cell lymphocytosis: a descriptive clinical study. Blood. 1994, 84: 2721-2725.
Rabbani GR, Phyliky RL, Tefferi A: A long-term study of patients with chronic natural killer cell lymphocytosis. Br J Haematol. 1999, 106: 960-966. 10.1046/j.1365-2141.1999.01624.x.
Martin MP, Nelson G, Lee JH, Pellett F, Gao X, Wade J, Wilson MJ, Trowsdale J, Gladman D, Carrington M: Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol. 2002, 169: 2818-2822.
van der Slik AR, Koeleman BP, Verduijn W, Bruining GJ, Roep BO, Giphart MJ: KIR in type 1 diabetes: disparate distribution of activating and inhibitory natural killer cell receptors in patients versus HLA-matched control subjects. Diabetes. 2003, 52: 2639-2642.
Takeno M, Shimoyama Y, Kashiwakura JI, Nagafuchi H, Sakane T, Suzuki N: Abnormal killer inhibitory receptor expression on natural killer cells in patients with Behcet's disease. Rheumatol Int. 2003, [epub ahead of print]
Namekawa T, Snyder MR, Yen JH, Goehring BE, Leibson PJ, Weyand CM, Goronzy JJ: Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J Immunol. 2000, 165: 1138-1145.
Warrington KJ, Takemura S, Goronzy JJ, Weyand CM: CD4+, CD28- T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001, 44: 13-20. 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6.
Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, Weyand CM, Goronzy JJ: Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med. 2001, 193: 1159-1168. 10.1084/jem.193.10.1159.
Snyder MR, Lucas M, Vivier E, Weyand CM, Goronzy JJ: Selective activation of the c-Jun NH2-terminal protein kinase signaling pathway by stimulatory KIR in the absence of KARAP/ DAP12 in CD4+ T cells. J Exp Med. 2003, 197: 437-449. 10.1084/jem.20020383.
Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T: Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci USA. 2003, 100: 9452-9457. 10.1073/pnas.1632807100.
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T: Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999, 285: 727-729. 10.1126/science.285.5428.727.
Tak PP, Kummer JA, Hack CE, Daha MR, Smeets TJ, Erkelens GW, Meinders AE, Kluin PM, Breedveld FC: Granzyme-positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum. 1994, 37: 1735-1743.
Dalbeth N, Callan MF: A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002, 46: 1763-1772. 10.1002/art.10410.
Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ: Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158negative phenotype. Rheumatology (Oxford). 2003, 42: 870-878. 10.1093/rheumatology/keg240.
Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA: Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001, 97: 3146-3151. 10.1182/blood.V97.10.3146.
Farag SS, VanDeusen JB, Fehniger TA, Caligiuri MA: Biology and clinical impact of human natural killer cells. Int J Hematol. 2003, 78: 7-17.
Fu**ami RS: Can virus infections trigger autoimmune disease?. J Autoimmun. 2001, 16: 229-234. 10.1006/jaut.2000.0484.
Paya CV, Patick AK, Leibson PJ, Rodriguez M: Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler's virus. J Immunol. 1989, 143: 95-102.
Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR: From infection to autoimmunity. J Autoimmun. 2001, 16: 175-186. 10.1006/jaut.2000.0492.
Takeda K, Dennert G: The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993, 177: 155-164. 10.1084/jem.177.1.155.
Smeltz RB, Wolf NA, Swanborg RH: Inhibition of autoimmune T cell responses in the DA rat by bone marrow-derived NK cells in vitro: implications for autoimmunity. J Immunol. 1999, 163: 1390-1397.
Fort MM, Leach MW, Rennick DM: A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J Immunol. 1998, 161: 3256-3261.
Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T: Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997, 186: 1677-1687. 10.1084/jem.186.10.1677.
Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N: Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998, 28: 1681-1688. 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T.
Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG: IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000, 165: 3099-3104.
Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A: Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999, 163: 4647-4650.
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G: Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002, 195: 327-333. 10.1084/jem.20010938.
Piccioli D, Sbrana S, Melandri E, Valiante NM: Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002, 195: 335-341. 10.1084/jem.20010934.
Moretta A: Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002, 2: 957-964. 10.1038/nri956.
Schott E, Bonasio R, Ploegh HL: Elimination in vivo of develo** T cells by natural killer cells. J Exp Med. 2003, 198: 1213-1224. 10.1084/jem.20030918.
Shi FD, Wang HB, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren HG: Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nat Immunol. 2000, 1: 245-251. 10.1038/79792.
Korsgren M, Persson CG, Sundler F, Bjerke T, Hansson T, Chambers BJ, Hong S, Van Kaer L, Ljunggren HG, Korsgren O: Natural killer cells determine the development of allergen-induced eosinophilic airway inflammation in mice. J Exp Med. 1999, 189: 553-562. 10.1084/jem.189.3.553.
Backstrom E, Chambers BJ, Kristensson K, Ljunggren HG: Direct NK cell-mediated lysis of syngenic dorsal root ganglia neurons in vitro. J Immunol. 2000, 165: 4895-4900.
Morse RH, Seguin R, McCrea EL, Antel JP: NK cell-mediated lysis of autologous human oligodendrocytes. J Neuroimmunol. 2001, 116: 107-115. 10.1016/S0165-5728(01)00289-2.
Hickey WF, Ueno K, Hiserodt JC, Schmidt RE: Exogenously-induced, natural killer cell-mediated neuronal killing: a novel pathogenetic mechanism. J Exp Med. 1992, 176: 811-817. 10.1084/jem.176.3.811.
Backstrom E, Chambers BJ, Ho EL, Naidenko OV, Mariotti R, Fremont DH, Yokoyama WM, Kristensson K, Ljunggren HG: Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions. Eur J Immunol. 2003, 33: 92-100. 10.1002/immu.200390012.
Kitaichi N, Kotake S, Morohashi T, Onoe K, Ohno S, Taylor AW: Diminution of experimental autoimmune uveoretinitis (EAU) in mice depleted of NK cells. J Leukoc Biol. 2002, 72: 1117-1121.
Flodstrom M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N: Target cell defense prevents the development of diabetes after viral infection. Nat Immunol. 2002, 3: 373-382. 10.1038/ni771.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
French, A.R., Yokoyama, W.M. Natural killer cells and autoimmunity. Arthritis Res Ther 6, 8 (2003). https://doi.org/10.1186/ar1034
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar1034