Abstract
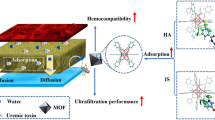
In this study, a novel copper ion (Cu2+) crosslinked composites beads with components of sodium alginate (SA) as gel matrix and UiO-66-(COOH)2 metal organic framework (MOF) nanoparticle as functional filler could effectively adsorb toxins in dialysate. The maximum adsorption capacity of the optimized composite beads (Cu-SA/U-4) for creatinine reached 125.0 mg/g with a positive synergistic effect based on Langmuir isotherm model and pseudo-second-order kinetic model, which was 1.75 times and 1.50 times that of single component beads (Cu-SA) and UiO-66-(COOH)2 nanoparticle respectively. Furthermore, when the dialysis performance using thin-film nanofibrous composite (TFNC) membrane was comparable, introduction of Cu-SA/U-4 beads into the dialysate allowed the volume of dialysate reduced to one-tenth of that without beads. Therefore, the coordination of such highly efficient adsorbent beads and TFNC dialysis membrane can economize the usage of the dialysate and contributes to realize lightweight hemodialysis. More importantly, we not only prepared a kind of composite bead via carefully designed strategy, but also proposed widely applicable idea of using adsorbents in combination with dialysis membrane to realize lightweight or miniaturized hemodialysis.
Graphical Abstract














Similar content being viewed by others
References
Legallais C, Catapano G, von Harten B, Baurmeister U. A theoretical model to predict the in vitro performance of hemodiafilters. J Membr Sci 2000;168:3.
Shao G, Zang Y, Hinds BJ. TiO2 nanowires based system for urea photodecomposition and dialysate regeneration. ACS Appl Nano Mater 2019;2:6116.
Koubaissy B, Toufaily J, Yaseen Z, Daou TJ, Jradi S, Hamieh T. Adsorption of uremic toxins over dealuminated zeolites. Adsorpt Sci Technol 2016;35:3.
Cao Y, Gu Y, Wang K, Wang X, Gu Z, Ambrico T, Castro MA, Lee J, Gibbons W, Rice JA. Adsorption of creatinine on active carbons with nitric acid hydrothermal modification. J Taiwan Inst Chem Eng 2016;66:347.
Benbakhta G, Mokhtari M, Hamaizi H, Galera MM, Garcí MDG. Extraction of creatinine by adsorption onto pure micro- and mesoporous silica materials. J Biotech Res 2017;8:113.
Zhao Q, Seredych M, Precetti E, Shuck CE, Harhay M, Pang R, Shan CX, Gogotsi Y. Adsorption of uremic toxins using Ti3C2Tx MXene for dialysate regeneration. ACS Nano 2020;14:11787.
Wu X, Sun X, Pan M, Liu H. Sustained drug release from chitosan@alginate microspheres with porous core and closed outer surface pore structure. J Controlled Release 2017;259:e5.
Kumar A, Rao KM, Han SS. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem Eng J 2017;317:119.
Shan S, Tang H, Zhao Y, Wang W, Cui F. Highly porous zirconium-crosslinked graphene oxide/alginate aerogel beads for enhanced phosphate removal. Chem Eng J 2019;359:779.
Ma H, Pu S, Ma J, Yan C, Zinchenko A, Pei X, Chu W. Formation of multi-layered chitosan honeycomb spheres via breath-figure-like approach in combination with co-precipitation processing. Mater Lett 2018;211:91.
Meng R, Liu L, ** Y, Luo Z, Gao H, Yao J. Recyclable carboxylated cellulose beads with tunable pore structure and size for highly efficient dye removal. Cellulose 2019;26:8963.
Wang K, Ma H, Pu S, Yan C, Wang M, Yu J, Wang X, Chu W, Zinchenko A. Hybrid porous magnetic bentonite-chitosan beads for selective removal of radioactive cesium in water. J Hazard Mater 2019;362:160.
Lessa EF, Medina AL, Ribeiro AS, Fajardo AR. Removal of multi-metals from water using reusable pectin/cellulose microfibers composite beads. Arabian J Chem 2020;13:709.
Mokhtar A, Abdelkrim S, Djelad A, Sardi A, Boukoussa B, Sassi M, Bengueddach A. Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohydr Polym 2020;229:115399.
Zhao F, Wang Q, Dong J, **an M, Yu J, Yin H, Chang Z, Mu X, Hou T, Wang J. Enzyme-inorganic nanoflowers/alginate microbeads: An enzyme immobilization system and its potential application. Process Biochem 2017;57:87.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012;37:106.
Li L, Zhao J, Sun Y, Yu F, Ma J. Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem Eng J 2019;372:1091.
Vicini S, Castellano M, Mauri M, Marsano E. Gelling process for sodium alginate: new technical approach by using calcium rich micro-spheres. Carbohydr Polym 2015;134:767.
Sinha P, Ubaidulla U, Hasnain MS, Nayak AK, Rama B. Alginate-okra gum blend beads of diclofenac sodium from aqueous template using ZnSO4 as a cross-linker. Int J Biol Macromol 2015;79:555.
Qu P, Li Y, Huang H, Chen J, Yu Z, Huang J, Wang H, Gao B. Urea formaldehyde modified alginate beads with improved stability and enhanced removal of Pb2+, Cd2+, and Cu2+. J Hazard Mater 2020;396:122664.
Hu X, Long L, Gong T, Zhang J, Yan J, Xue Y. Enhanced alginate-based microsphere with the pore-forming agent for efficient removal of Cu (II). Chemosphere 2020;240:124860.
E T, Ma D, Yang S, Hao X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J Alloys Compd 2020;832:154833.
Hu Y, Wei X, Hu Y, Wang W, Fan J, Liu X, Chai W, Zhou Z, Ren Z. Facile preparation of sodium alginate-based gel spheres by droplet polymerization method for removal of levofloxacin from aqueous solution. Chem Eng J 2020;392:123718.
Kalasin S, Sangnuang P, Khownarumit P, Tang IM, Surareungchai W. Evidence of Cu(I) coupling with creatinine using cuprous nanoparticles encapsulated with polyacrylic acid gel-Cu(II) in facilitating the determination of advanced kidney dysfunctions. ACS Biomater Sci Eng 2020;6:1247.
Hua W, Zhang T, Wang M, Zhu Y, Wang X. Hierarchically structural PAN/UiO-66-(COOH)2 nanofibrous membranes for effective recovery of Terbium(III) and Europium(III) ions and their photoluminescence performances. Chem Eng J 2019;370:729.
Yu X, Shen L, Zhu Y, Li X, Yang Y, Wang X, Zhu M, Hsiao BS. High performance thin-film nanofibrous composite hemodialysis membranes with efficient middle-molecule uremic toxin removal. J Membr Sci 2017;523:173.
Li Q, Ding C, Yuan W, **e R, Zhou X, Zhao Y, Yu M, Yang Z, Sun J, Tian Q, Han F, Li H, Deng X, Li G, Liu Z. Highly stretchable and permeable conductors based on shrinkable electrospun fiber mats. Adv Fiber Mater 2021;3:302.
Wang L, Fu Q, Yu J, Liu L, Ding B. Cellulose nanofibrous membranes modified with phenyl glycidyl ether for efficient adsorption of bovine serum albumin. Adv Fiber Mater 2019;1:188.
Yang X, Li L, Yang D, Nie J, Ma G. Electrospun core-shell fibrous 2D scaffold with biocompatible poly(glycerol sebacate) and poly-l-lactic acid for wound healing. Adv Fiber Mater 2020;2:105.
Li P, Cheng C, Shen K, Zhang T, Wang X, Hsiao BS. Enhancing dehydration performance of isopropanol by introducing intermediate layer into sodium alginate nanofibrous composite pervaporation membrane. Adv Fiber Mater 2019;1:137.
Yu X, Zhu Y, Cheng C, Zhang T, Wang X, Hsiao BS. Novel thin-film nanofibrous composite membranes containing directional toxin transport nanochannels for efficient and safe hemodialysis application. J Membr Sci 2019;582:151.
Zhu Y, Yu X, Zhang T, Hua W, Wang X. Nanofibrous composite hemodiafiltration membrane: a facile approach towards tuning the barrier layer for enhanced performance. Appl Surf Sci 2019;465:950.
Zhu Y, Yu X, Zhang T, Li P, Wang X. Biomimetic sulfated silk nanofibrils for constructing rapid mid-molecule toxins removal nanochannels. J Membr Sci 2020;598:117667.
Zhang X, Hu Q, **a T, Zhang J, Yang Y, Cui Y, Chen B, Qian G. Turn-on and ratiometric luminescent sensing of hydrogen sulfide based on metal-organic frameworks. ACS Appl Mater Inter 2016;8:32259.
Zhu Z, Wu P, Liu G, He X, Qi B, Zeng G, Wang W, Sun Y, Cui F. Ultrahigh adsorption capacity of anionic dyes with sharp selectivity through the cationic charged hybrid nanofibrous membranes. Chem Eng J 2017;313:957.
Feng Y, Wang Y, Wang Y, Zhang XF, Yao J. In-situ gelation of sodium alginate supported on melamine sponge for efficient removal of copper ions. J Colloid Interf Sci 2018;512:7.
Yang JH, Lei ZJ, Dai YH, Luo Y, **e SB, Wang JS, Zhou SK, Wei B, Li C, Hu SQ. Preparation of aluminum sludge composite gel spheres and adsorption of U(IV) from aqueous solution. Environ Sci Pollut Res 2020;27:26835.
Lu L, Samarasekera C, Yeow JTW. Creatinine adsorption capacity of electrospun polyacrylonitrile (PAN)-zeolite nanofiber membranes for potential artificial kidney applications. J Appl Polym Sci 2015;132:42418.
Ti**k MS, Wester M, Sun J, Saris A, Bolhuis-Versteeg LA, Saiful S, Joles JA, Borneman Z, Wessling M, Stamatialis DF. A novel approach for blood purification: mixed-matrix membranes combining diffusion and adsorption in one step. Acta Biomater 2012;8:2279.
Gao BJ, Yang Y, Wang J, Zhang Y. Preparation and adsorption characteristic of polymeric microsphere with strong adsorbability for creatinine. J Biochem Mol Toxic 2008;22:166.
Namekawa K, Tokoro Schreiber M, Aoyagi T, Ebara M. Fabrication of zeolite–polymer composite nanofibers for removal of uremic toxins from kidney failure patients. Biomater Sci 2014;2:674.
Ye C, Gong Q, Lu F, Liang J. Adsorption of uraemic toxins on carbon nanotubes. Sep Purif Technol 2007;58:2.
Bergé-Lefranc D, Pizzala H, Denoyel R, Hornebecq V, Bergé-Lefranc J-L, Guieu R, Brunet P, Ghobarkar H, Schäf O. Mechanism of creatinine adsorption from physiological solutions onto mordenite. Micropor Mesopor Mat 2009;119:186.
Yu J, Wu Y, Wang S, Ma X. The preparation of cellulose nitrate derivatives and their adsorption properties for creatinine. Carbohydr Polym 2007;70:8.
Ding S, Zhang T, Li P, Wang X. Dialysis/adsorption bifunctional thin-film nanofibrous composite membrane for creatinine clearance in portable artificial kidney. J Membr Sci 2021;636:119550.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2232020A-04), Shanghai Municipal Natural Science Foundation (19ZR1401300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, S., Li, P., Zhang, T. et al. Coordination of Copper Ion Crosslinked Composite Beads with Enhanced Toxins Adsorption and Thin-Film Nanofibrous Composite Membrane for Realizing the Lightweight Hemodialysis. Adv. Fiber Mater. 4, 556–570 (2022). https://doi.org/10.1007/s42765-021-00131-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-021-00131-6