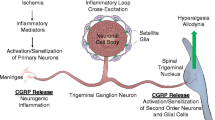
Abstract
Calcitonin gene-related peptide (CGRP) is known to be involved in the pathophysiology of migraine headache. CGRP may play a role in the mechanisms of somatic, visceral, neuropathic, and inflammatory pain. Currently, there are multiple new biologic therapies targeting CGRP which have been approved for the acute and preventive treatment of migraine. Studies have also shown CGRP to be involved in the pathophysiology of trigeminal neuralgia (TN), an often severe and refractory facial pain condition. This article outlines the pathophysiology of trigeminal neuralgia and the role CGRP may play in this condition. We discuss the current evidence on CGRP in trigeminal neuralgia and how modulation of CGRP may be a potential target in the treatment of trigeminal neuralgia in the future. With many similarities in the pathophysiology and efficacy of drug therapies for migraine and trigeminal neuralgia, we hypothesize that targeting CGRP may be a potential mechanism of pain relief in trigeminal neuralgia.


Similar content being viewed by others
References
Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol. 1990;27(1):89–95. https://doi.org/10.1002/ana.410270114.
De Toledo IP, Conti Réus J, Fernandes M, Porporatti AL, Peres MA, Takaschima A, Linhares MN, De Luca Canto G. Prevalence of trigeminal neuralgia: a systematic review. J Am Dent Assoc. 2016;147(7):570–576.e572. https://doi.org/10.1016/j.adaj.2016.02.014.
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd. (2018). Cephalalgia., 38(1), 1-211. https://doi.org/10.1177/0333102417738202.
Jones MR, Urits I, Ehrhardt KP, Cefalu JN, Kendrick JB, Park DJ, Cornett EM, Kaye AD, Viswanath O. A comprehensive review of trigeminal neuralgia. Curr Pain Headache Rep. 2019;23(10):74. https://doi.org/10.1007/s11916-019-0810-0.
Rasmussen P. Facial pain. II. A prospective survey of 1052 patients with a view of: character of the attacks, onset, course, and character of pain. Acta Neurochir. (Wien). 1990;107(3-4):121–8. https://doi.org/10.1007/BF01405790.
Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Concomitant persistent pain in classical trigeminal neuralgia--evidence for different subtypes. Headache. 2014a;54(7):1173–83. https://doi.org/10.1111/head.12384.
Brisman R. Constant face pain in typical trigeminal neuralgia and response to γ knife radiosurgery. Stereotact Funct Neurosurg. 2013;91(2):122–8. https://doi.org/10.1159/000343206.
Cruccu G, Finnerup NB, Jensen TS, Scholz J, Sindou M, Svensson P, Treede RD, Nurmikko T. Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology. 2016;87(2):220–8. https://doi.org/10.1212/WNL.0000000000002840.
Brisman R. Trigeminal neuralgia and multiple sclerosis. Arch Neurol. 1987;44(4):379–81. https://doi.org/10.1001/archneur.1987.00520160021008.
Di Stefano G, Maarbjerg S, Truini A. Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. J Headache Pain. 2019;20(1):20. https://doi.org/10.1186/s10194-019-0969-0.
Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K, Nurmikko T, Zakrzewska JM. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71(15):1183–90. https://doi.org/10.1212/01.wnl.0000326598.83183.04.
Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia--a prospective systematic study of clinical characteristics in 158 patients. Headache. 2014b;54(10):1574–82. https://doi.org/10.1111/head.12441.
Cruccu G. Trigeminal neuralgia. CONTINUUM: Lifelong Learn. in Neurol. 2017;23(2):396–420. https://doi.org/10.1212/CON.0000000000000451.
Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K, Nurmikko T, Zakrzewska JM. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013–28. https://doi.org/10.1111/j.1468-1331.2008.02185.x.
Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T, Obermann M, Cruccu G, Maarbjerg S. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020;19(9):784–96. https://doi.org/10.1016/S1474-4422(20)30233-7.
McQuay HJ, Moore RA, Eccleston C, Morley S, Williams AC. Systematic review of outpatient services for chronic pain control. Health Technol Assess. 1997;1(6):i–iv. https://doi.org/10.3310/hta1060.
Al-Quliti KW. Update on neuropathic pain treatment for trigeminal neuralgia: The pharmacological and surgical options. Neurosciences. (Riyadh). 2015;20(2):107–14. https://doi.org/10.17712/nsj.2015.2.20140501.
Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015;11:289–99. https://doi.org/10.2147/TCRM.S37592.
Obermann M. Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord. 2010;3(2):107–15. https://doi.org/10.1177/1756285609359317.
Zhang WB, Min LZ, Tao BB, Sun QY, Li ST, Wang XQ. Prognosis comparison of different branches of trigeminal neuralgia. World Neurosurg. 2020;133:e1–5. https://doi.org/10.1016/j.wneu.2019.06.115.
Zhang WB, Zeng YY, Chang BW, Min LZ, Sun QY, Li B, Tao BB, Wang XQ. Prognostic nomogram for microvascular decompression-treated trigeminal neuralgia. Neurosurg Rev. 2021;44(1):571–7. https://doi.org/10.1007/s10143-020-01251-0.
Zheng JH, Sun K, Zhang HT, **e YJ, Wang-Yang LX, Chen HY, Wang C. A study on the recurrence rate of trigeminal neuralgia after MVD and the related factors. J Neurol Surg B Skull Base. 2020;81(5):572–8. https://doi.org/10.1055/s-0039-1692687.
Mimeh H, Fenech Magrin AM, Myers S, Ghanem AM. A critical review of botulinum toxin type a in the prophylactic treatment of chronic migraine in adults. Aesthet Surg J. 2019;39(8):898–907. https://doi.org/10.1093/asj/sjy224.
Tassorelli C, Sances G, Avenali M, De Icco R, Martinelli D, Bitetto V, Nappi G, Sandrini G. Botulinum toxin for chronic migraine: clinical trials and technical aspects. Toxicon. 2018;147:111–5. https://doi.org/10.1016/j.toxicon.2017.08.026.
Morra ME, Elgebaly A, Elmaraezy A, Khalil AM, Altibi AM, Vu TL, Mostafa MR, Huy NT, Hirayama K. Therapeutic efficacy and safety of botulinum toxin A therapy in trigeminal neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain. 2016;17(1):63. https://doi.org/10.1186/s10194-016-0651-8.
Zhang Y, Lian Y, Zhang H, **e N, Chen Y. CGRP plasma levels decrease in classical trigeminal neuralgia patients treated with botulinum toxin type A: a pilot study. Pain Med. 2020;21(8):1611–5. https://doi.org/10.1093/pm/pnaa028.
Kanai A, Saito M, Hoka S. Subcutaneous sumatriptan for refractory trigeminal neuralgia. Headache. 2006;46(4):577–82. https://doi.org/10.1111/j.1526-4610.2006.00405.x.
Kanai A, Suzuki A, Osawa S, Hoka S. Sumatriptan alleviates pain in patients with trigeminal neuralgia. Clin J Pain. 2006;22(8):677–80.
Shimohata K, Shimohata T, Motegi R, Miyashita K. Nasal sumatriptan as adjunctive therapy for idiopathic trigeminal neuralgia: report of three cases. Headache. 2009;49(5):768–70. https://doi.org/10.1111/j.1526-4610.2008.01254.x.
Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25(3):179–83. https://doi.org/10.1111/j.1468-2982.2005.00836.x.
Halker RB, Vargas BB. Pathophysiology of migraine & triptan mechanism of action. In: Triptans for Migraine. London, England: Future Medicine Ltd; 2012. p. 6–15.
Benemei S, Cortese F, Labastida-Ramírez A, Marchese F, Pellesi L, Romoli M, Vollesen AL, Lampl C, Ashina M. Triptans and CGRP blockade - impact on the cranial vasculature. J Headache Pain. 2017;18(1):103. https://doi.org/10.1186/s10194-017-0811-5.
Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298(5871):240–4. https://doi.org/10.1038/298240a0.
Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6(10):573–82. https://doi.org/10.1038/nrneurol.2010.127.
Tepper SJ. History and review of anti-calcitonin gene-related peptide (CGRP) therapies: from translational research to treatment. Headache. 2018;58(Suppl 3):238–75. https://doi.org/10.1111/head.13379.
Yuan H, Spare NM, Silberstein SD. Targeting CGRP for the prevention of migraine and cluster headache: a narrative review. Headache. 2019;59(Suppl 2):20–32. https://doi.org/10.1111/head.13583.
Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain. 2017;18(1):34. https://doi.org/10.1186/s10194-017-0741-2.
Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622. https://doi.org/10.1152/physrev.00034.2015.
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain. 2013;14(11):1289–303. https://doi.org/10.1016/j.jpain.2013.03.010.
Edvinsson L, Fredholm BB, Hamel E, Jansen I, Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1985;58(2):213–7. https://doi.org/10.1016/0304-3940(85)90166-1.
Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache. 2012;52(9):1411–27. https://doi.org/10.1111/j.1526-4610.2012.02212.x.
Messlinger K. The big CGRP flood - sources, sinks and signalling sites in the trigeminovascular system. J Headache Pain. 2018;19(1):22. https://doi.org/10.1186/s10194-018-0848-0.
Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci. 2011;31(40):14204–17. https://doi.org/10.1523/JNEUROSCI.3285-11.2011.
Lennerz JK, Rühle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507(3):1277–99. https://doi.org/10.1002/cne.21607.
Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015;1600:93–109. https://doi.org/10.1016/j.brainres.2014.11.031.
Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol. 1981;65:1–111. https://doi.org/10.1007/978-3-642-67750-2.
Pannese E, Ledda M, Cherkas PS, Huang TY, Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat Embryol. (Berl). 2003;206(5):337–47. https://doi.org/10.1007/s00429-002-0301-6.
Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48(3):457–76. https://doi.org/10.1016/j.brainresrev.2004.09.001.
Vit JP, Jasmin L, Bhargava A, Ohara PT. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006;2(4):247–57. https://doi.org/10.1017/s1740925x07000427.
Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47(7):1008–23. https://doi.org/10.1111/j.1526-4610.2007.00854.x.
Afroz S, Arakaki R, Iwasa T, Oshima M, Hosoki M, Inoue M, Baba O, Okayama Y, Matsuka Y. CGRP induces differential regulation of cytokines from satellite glial cells in trigeminal ganglia and orofacial nociception. Int J Mol Sci. 2019;20(3):711. https://doi.org/10.3390/ijms20030711.
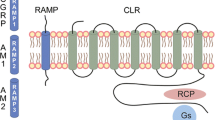
Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109(1-2):173–97. https://doi.org/10.1016/j.pharmthera.2005.06.015.
Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol. 2013;170(7):1293–307. https://doi.org/10.1111/bph.12129.
Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278(5):3293–7. https://doi.org/10.1074/jbc.C200629200.
Walker CS, Eftekhari S, Bower RL, Wilderman A, Insel PA, Edvinsson L, Waldvogel HJ, Jamaluddin MA, Russo AF, Hay DL. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol. 2015;2(6):595–608. https://doi.org/10.1002/acn3.197.
Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347–60. https://doi.org/10.1093/brain/124.12.2347.
Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 2017;37(7):648–57. https://doi.org/10.1177/0333102416687280.
Rappaport HZ, Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain. 1994;56(2):127–38. https://doi.org/10.1016/0304-3959(94)90086-8.
Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18(1):4–13. https://doi.org/10.1097/00002508-200201000-00002.
Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 2020;383(8):754–62. https://doi.org/10.1056/NEJMra1914484.
Tajti J, Uddman R, Möller S, Sundler F, Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst. 1999;76(2-3):176–83. https://doi.org/10.1016/s0165-1838(99)00024-7.
Ashina M. Migraine. N Engl J Med. 2020;383(19):1866–76. https://doi.org/10.1056/NEJMra1915327.
Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–7. https://doi.org/10.1002/ana.410280213.
van Dongen RM, Zielman R, Noga M, Dekkers OM, Hankemeier T, van den Maagdenberg AM, Terwindt GM, Ferrari MD. Migraine biomarkers in cerebrospinal fluid: a systematic review and meta-analysis. Cephalalgia. 2017;37(1):49–63. https://doi.org/10.1177/0333102415625614.
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. https://doi.org/10.1046/j.1468-2982.2002.00310.x.
Edvinsson L. The trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache. 2017;57(Suppl 2):47–55. https://doi.org/10.1111/head.13081.
Limmroth V, Katsarava Z, Liedert B, Guehring H, Schmitz K, Diener HC, Michel MC. An in vivo rat model to study calcitonin gene related peptide release following activation of the trigeminal vascular system. Pain. 2001;92(1-2):101–6. https://doi.org/10.1016/s0304-3959(00)00475-9.
Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–59. https://doi.org/10.1097/j.pain.0000000000000831.
Qin ZL, Yang LQ, Li N, Yue JN, Wu BS, Tang YZ, Guo YN, Lai GH, Ni JX. Clinical study of cerebrospinal fluid neuropeptides in patients with primary trigeminal neuralgia. Clin Neurol Neurosurg. 2016;143:111–5. https://doi.org/10.1016/j.clineuro.2016.02.012.
Michot B, Bourgoin S, Viguier F, Hamon M, Kayser V. Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain. 2012;153(9):1939–48. https://doi.org/10.1016/j.pain.2012.06.009.
Michot B, Kayser V, Hamon M, Bourgoin S. CGRP receptor blockade by MK-8825 alleviates allodynia in infraorbital nerve-ligated rats. Eur J Pain. 2015;19(2):281–90. https://doi.org/10.1002/ejp.616.
**ong W, Wu RP, Tan MX, Tong ZJ, He LK, Guan S, Liu LJ, Yin CC, Shen YL, Ge HX, Gao Y. Emodin inhibits the expression of receptor and calcitonin-gene-related peptide release in trigeminal ganglia of trigeminal neuralgia rats. Int J Clin Exp Pathol. 2017;10(11):11317–25.
Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–5. https://doi.org/10.1016/s0166-2236(00)01854-3.
Yang YJ, Hu L, **a YP, Jiang CY, Miao C, Yang CQ, Yuan M, Wang L. Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J Neuroinflammation. 2016;13(1):84. https://doi.org/10.1186/s12974-016-0550-6.
Parascandolo E, Levinson K, Rizzoli P, Sharon R. Efficacy of erenumab in the treatment of trigeminal neuralgia: a retrospective case series. Neurol: Clin Pract. 2021;11(3):227–31. https://doi.org/10.1212/cpj.0000000000001075.
Schott Andersen AS, Maarbjerg S, Noory N, Heinskou TB, Forman JL, Cruccu G, Ashina M, Bendtsen L. Safety and efficacy of erenumab in patients with trigeminal neuralgia in Denmark: a double-blind, randomised, placebo-controlled, proof-of-concept study. Lancet Neurol. 2022;21(11):994–1003. https://doi.org/10.1016/S1474-4422(22)00294-0.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Roni Sharon, M. D., identified the idea for this work. Rachel Retsky, B. S., conducted the literature search and wrote the first draft of the manuscript. Roni Sharon, M. D.; Sait Ashina, M. D.; Daniel Oved, M. D.; and Rachel Retsky, B. S., edited the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Sait Ashina (S. A.) received honoraria for consulting from Allergan/AbbVie, Amgen, Biohaven, Eli Lilly, Impel, NeuroPharma, Novartis, Satsuma, Supernus, Theranica, and Percept. Roni Sharon has been a paid speaker or consultant for the following companies: Novartis, Abbvie, Pfizer, Teva, Eli Lilly, Theranica, Neurolief, BOL, GLG, Guidepoint. Rachel Retsky and Daniel Oved declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Retsky, R., Ashina, S., Oved, D. et al. Calcitonin Gene-Related Peptide and Trigeminal Neuralgia. SN Compr. Clin. Med. 5, 75 (2023). https://doi.org/10.1007/s42399-023-01407-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-023-01407-1