Abstract
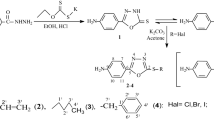
A series of Schiff base compounds were synthesized by the reaction of different 3,5-dihalosalicylaldehyde (halo atoms equal to Cl, Br and I) with polymethylenediamines of varying chain length. The Schiff bases were characterized using FT-IR, UV–Vis, 1H NMR, 13C NMR and mass spectroscopic techniques, and elemental analyses (CHN), and crystal structure of some compounds was determined by X-ray crystallography. The in vitro biological screening effects of the synthesized compounds were tested against different microbial kinds. The results revealed that all compounds were biologically active.







Similar content being viewed by others
References
M.J. O’Donnell, Acc. Chem. Res. 37, 506 (2004)
S. Rana, S.K. Mittal, N. Singh, J. Singh, C.E. Banks, Sens. Actuators B Chem. 239, 17 (2016)
G. Yuan, Y. Tian, J. Liu, H. Tu, J. Liao, J. Yang, Y. Yang, D. Wang, N. Liu, Chem. Eng. J. 326, 691 (2017)
A.A. Isse, A. Gennaro, E. Vianello, Electrochim. Acta 42, 2065 (1997)
P. Taylor, Synth. React. Inorg. Met. Chem. 32, 1729 (2002)
R.K. Parashar, R.C. Sharma, A. Kumar, G. Mohan, Inorg. Chim. Acta 151, 201 (1988)
S. Chandra, S. Gautam, H.K. Rajor, R. Bhatia, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 137, 749 (2015)
H. Khojasteh, V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, J. Iran. Chem. Soc. 14, 1139 (2017)
M. Hatefi Ardakani, M. Moghadam, S. Saeednia, Z. Pakdin-Parizi, J. Iran. Chem. Soc. 13, 631 (2016)
T. Jeewoth, H. Li Kam Wah, M.G. Bhowon, D. Ghoorohoo, K. Babooram, Synth. React. Inorg. Met. Chem. 30, 1023 (2000)
L. Shi, H.-M. Ge, S.-H. Tan, H.-Q. Li, Y.-C. Song, H.-L. Zhu, R.-X. Tan, Eur. J. Med. Chem. 42, 558 (2007)
H.G. Aslan, S. Akkoç, Z. Kökbudak, L. Aydın, J. Iran. Chem. Soc. 14, 2263 (2017)
Z. Guo, R. **ng, S. Liu, Z. Zhong, X. Ji, L. Wang, P. Li, Carbohydr. Res. 342, 1329 (2007)
M.M. Ali, M. Jesmin, J. Natl. Sci. Found. Sri Lanka 38, 145 (2010)
H. Khanmohammadi, M. Salehifard, M.H. Abnosi, J. Iran. Chem. Soc. 6, 300 (2009)
K. Neelima, S. Poonia, M. Siddiqui, D. Arshad, Kumar. Spectrochim. Acta A Mol. Biomol. Spectrosc. 155, 146 (2016)
T. Aboul-Fadl, F.A.S. Bin-Jubair, O. Aboul-Wafa, Eur. J. Med. Chem. 45, 4578 (2010)
M.J. Hearn, M.H. Cynamon, M.F. Chen, R. Coppins, J. Davis, H. Joo-On Kang, A. Noble, B. Tu-Sekine, M.S. Terrot, D. Trombino, M. Thai, E.R. Webster, R. Wilson, Eur. J. Med. Chem. 44, 4169 (2009)
K.S. Kumar, S. Ganguly, R. Veerasamy, E. De Clercq, Eur. J. Med. Chem. 45, 5474 (2010)
M.S. Alam, J.H. Choi, D.U. Lee, Bioorg. Med. Chem. 20, 4103 (2012)
A. Noureen, S. Saleem, T. Fatima, H.M. Siddiqi, B. Mirza, Pak. J. Pharm. Sci. 26, 113 (2013)
P. Paneerselvam, T. Raj, M.P.S. Ishar, B. Singh, V.D. Sharma, B.A. Rather, Indian J. Pharm. Sci. 72, 375 (2010)
M. Verma, S.N. Pandeya, K.N. Singh, J.P. Stables, Acta Pharm. 54, 49 (2004)
S.E. Harpstrite, S.D. Collins, A. Oksman, D.E. Goldberg, V. Sharma, Med. Chem. 4, 392 (2008)
C. Liang, J. **a, D. Lei, X. Li, Q. Yao, J. Gao, Eur. J. Med. Chem. 74, 742 (2013)
Z. Asadi, M. Asadi, F. Dehghani Firuzabadi, R. Yousefi, M. Jamshidi, J. Iran. Chem. Soc. 11, 423 (2014)
S.P. Xu, L. Shi, Y. Pei, Y. Yang, G. Xu, H.L. Zhu, J. Coord. Chem. 63, 3463 (2010)
H. Kargar, Transit. Met. Chem. 39, 811 (2014)
Bruker and W. AXS Programs: SMART, version 5.625; SAINT, version 6.45; SADABS, version 2.10; XPREP, version 6.14. Bruker AXS Inc.: Madison, (2003)
G.M. Sheldrick, Acta Crystallogr. A 64, 112 (2008)
S. Al, Acta Crystallogr D 65, 148 (2009)
B. Ambrozini, E. Dockal, É. Cavalheiro, J. Therm. Anal. 115, 979 (2014)
D. Gürbüz, A. Cinarli, A. Tavman, A.S. Birteksz, Chin. J. Chem. 30, 970 (2012)
Y. Cui, X. Dong, Y. Li, Z. Li, W. Chen, Eur. J. Med. Chem. 58, 323 (2012)
M. Yıldız, Ö. Karpuz, C.T. Zeyrek, B. Boyacıoğlu, H. Dal, N. Demir, N. Yıldırım, H. Ünver, J. Mol. Struct. 1094, 148 (2015)
M. Asadi, H. Sepehrpour, K. Mohammadi, J. Serbian Chem. Soc. 76, 63 (2011)
H.H. Hammud, A. Ghannoum, M.S. Masoud, Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 63, 255 (2006)
A. Cinarli, D. Gürbüz, A. Tavman, A. Seher Birteksöz, Bull. Chem. Soc. Ethiop. 25, 407 (2011)
E.G. Bakirdere, M.F. Fellah, M. Kaya, J. Serb. Chem. Soc. 81, 509 (2016)
M. Malathy, Int. J. Sci. Technol. 2, 157 (2014)
F. Bagheri, A. Olyaei, J. Serb. Chem. Soc. 81, 1111 (2016)
A. Golcu, M. Tumer, H. Demirelli, R.A. Wheatley, Inorg. Chim. Acta. 358, 1785 (2005)
A.N. Kursunlu, E. Guler, F. Sevgi, B. Ozkalp, J. Mol. Struct. 1048, 476 (2013)
S.P. Xu, L. Shi, P.C. Lv, X.L. Li, H.L. Zhu, J. Coord. Chem. 62, 3198 (2009)
Acknowledgements
The support of this work by Payame Noor University Council of Research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ardakani, A.A., Kargar, H., Feizi, N. et al. Synthesis, characterization, crystal structures and antibacterial activities of some Schiff bases with N2O2 donor sets. J IRAN CHEM SOC 15, 1495–1504 (2018). https://doi.org/10.1007/s13738-018-1347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1347-6