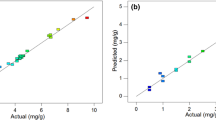
Abstract
Removal of arsenic (As), as a toxic, carcinogenic, and mutagenic water pollutant, has been a topic of much thought and discussion within environmental experts newly. One of the most popular AS removal method from aqueous solution is adsorption. To the best of our knowledge, the combination of ZnO/TiO2 immobilized on activated carbon (AC) has not been used to AS removal from water. Therefore the aims of this study are (i) to develop a novel immobilized ZnO/TiO2 activated carbon (I ZnO/TiO2 AC) for effective and economic arsenic removal, (ii) to investigate the effects of different variables (e.g., pH, contact time, dose) on I ZnO/TiO2 AC system efficiency, (iii) to achieve nonlinear modeling using response surface methodology (RSM) approach, and (iv) to optimize I ZnO/TiO2 AC system for As removal from water with RSM-based developed model. The final appropriate solution which was selected by developed response surface model demonstrated that the best dosage, pH, contact time, and initial concentration to reach permitted concentration for output (10 µg/L) are 5.187 g/L, 6.758, 287.574 min and 9.767 mg/L, respectively. The appropriate achieved desirability (0.996) depicted that the solution is acceptable.







Similar content being viewed by others
Data availability
The dataset and analyzed during the current study are available from the corresponding authors on realistic demand.
References
Pirsaheb M, Ejraei A (2016) Evaluating the performance of inorganic coagulants (Poly aluminum chloride, ferrous sulfate, ferric chloride and aluminum sulfate) in removing the turbidity from aqueous solutions. Int J Pharm Technol 8:13168–13181
Shokoohi R, Movahedian H, Dargahi A, Jafari AJ, Parvaresh A (2017) Survey on efficiency of BF/AS integrated biological system in phenol removal of wastewater. Desalin Water Treat 82:315–321
Samarghandi MR, Mohammadi M, Karami A, Tabandeh L, Dargahi A, Amirian F (2017) Residue analysis of pesticides, herbicides, and fungicides in various water sources using gas chromatography-mass detection. Pol J Environ Stud 26:2189–2195
Alalwan HA, Kadhom MA, Alminshid AH (2020) Removal of heavy metals from wastewater using agricultural byproducts. J Water Supply Res Technol AQUA 69:99–112
Dargahi A, Golestanifar H, Darvishi P, Karam A (2016) An investigation and comparison of removing heavy metals (lead and chromium) from aqueous solutions using magnesium oxide nanoparticles. Pol J Environ Stud 25:557–562
Almasi A, Dargahi A, Ahagh M, Janjani H, Mohammadi M, Tabandeh L (2016) Efficiency of a constructed wetland in controlling organic pollutants, nitrogen, and heavy metals from sewage. J Chem Pharm Sci 9:2924–2928
Ociński D, Jacukowicz-Sobala I, Mazur P, Raczyk J, Kociołek-Balawejder E (2016) Water treatment residuals containing iron and manganese oxides for arsenic removal from water–characterization of physicochemical properties and adsorption studies. Chem Eng J 294:210–221
Ahoranta SH, Kokko ME, Papirio S, Özkaya B, Puhakka JA (2016) Arsenic removal from acidic solutions with biogenic ferric precipitates. J Hazard Mater 306:124–132
Wang J, Xu W, Chen L, Huang X, Liu J (2014) Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem Eng J 251:25–34
Abejón A, Garea A, Irabien A (2015) Arsenic removal from drinking water by reverse osmosis: minimization of costs and energy consumption. Sep Purif Technol 144:46–53
Kumar PR, Chaudhari S, Khilar KC, Mahajan S (2004) Removal of arsenic from water by electrocoagulation. Chemosphere 55:1245–1252
Lee C-G, Alvarez PJ, Nam A, Park S-J, Do T, Choi U-S, Lee S-H (2017) Arsenic (V) removal using an amine-doped acrylic ion exchange fiber: kinetic, equilibrium, and regeneration studies. J Hazard Mater 325:223–229
**e L, Liu P, Zheng Z, Weng S, Huang J (2016) Morphology engineering of V2O5/TiO2 nanocomposites with enhanced visible light-driven photofunctions for arsenic removal. Appl Catal B 184:347–354
Samad A, Furukawa M, Katsumata H, Suzuki T, Kaneco S (2016) Photocatalytic oxidation and simultaneous removal of arsenite with CuO/ZnO photocatalyst. J Photochem Photobiol, A 325:97–103
Afsharnia M, Kianmehr M, Biglari H, Dargahi A, Karimi A (2018) Disinfection of dairy wastewater effluent through solar photocatalysis processes. Water Sci Eng 11:214–219
Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5:2782–2799
Mohanty D (2017) Conventional as well as emerging arsenic removal technologies—a critical review. Water Air Soil Pollut 228:381
Phanthasri J, Khamdahsag P, Jutaporn P, Sorachoti K, Wantala K, Tanboonchuy V (2018) Enhancement of arsenite removal using manganese oxide coupled with iron (III) trimesic. Appl Surf Sci 427:545–552
Vaiano V, Iervolino G, Rizzo L (2018) Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl Catal B 238:471–479
Samarghandi MR, Dargahi A, Zolghadr Nasab H, Ghahramani E, Salehi S (2020) Degradation of azo dye Acid Red 14 (AR14) from aqueous solution using H2O2/nZVI and S2O82–/nZVI processes in the presence of UV irradiation. Water Environ Res 92(8):1173–1183
Molinari R, Argurio P (2017) Arsenic removal from water by coupling photocatalysis and complexation-ultrafiltration processes: a preliminary study. Water Res 109:327–336
Guan X, Du J, Meng X, Sun Y, Sun B, Hu Q (2012) Application of titanium dioxide in arsenic removal from water: a review. J Hazard Mater 215:1–16
Nakajima T, Xu Y-H, Mori Y, Kishita M, Takanashi H, Maeda S, Ohki A (2005) Combined use of photocatalyst and adsorbent for the removal of inorganic arsenic (III) and organoarsenic compounds from aqueous media. J Hazard Mater 120:75–80
Zhang F-S, Itoh H (2006) Photocatalytic oxidation and removal of arsenite from water using slag-iron oxide-TiO2 adsorbent. Chemosphere 65:125–131
Xu Z, Meng X (2009) Size effects of nanocrystalline TiO2 on As (V) and As (III) adsorption and As (III) photooxidation. J Hazard Mater 168:747–752
Souza RP, Freitas TK, Domingues FS, Pezoti O, Ambrosio E, Ferrari-Lima AM, Garcia JC (2016) Photocatalytic activity of TiO2, ZnO and Nb2O5 applied to degradation of textile wastewater. J Photochem Photobiol, A 329:9–17
Kanakaraju D, Kockler J, Motti CA, Glass BD, Oelgemöller M (2015) Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl Catal B 166:45–55
Bo Zhong J, Zhang Li J, Mei Feng F, Lu Y, Zeng J, Hu W, Tang Z (2012) Improved photocatalytic performance of SiO2–TiO2 prepared with the assistance of SDBS. J Mol Catal A Chem 357:101–105
Hsieh W-P, Pan JR, Huang C, Su Y-C, Juang Y-J (2010) Enhance the photocatalytic activity for the degradation of organic contaminants in water by incorporating TiO2 with zero-valent iron. Sci Total Environ 408:672–679
Manzoli M, Chiorino A, Boccuzzi F (2005) Decomposition and combined reforming of methanol to hydrogen: a FTIR and QMS study on Cu and Au catalysts supported on ZnO and TiO2. Appl Catal B 57:201–209
Filipe P, Silva J, Silva R, De Castro JC, Gomes MM, Alves L, Santus R, Pinheiro T (2009) Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol Physiol 22:266–275
Liao S, Donggen H, Yu D, Su Y, Yuan G (2004) Preparation and characterization of ZnO/TiO2, SO42−/ZnO/TiO2 photocatalyst and their photocatalysis. J Photochem Photobiol, A 168:7–13
Wang J, Li J, **e Y, Li C, Han G, Zhang L, Xu R, Zhang X (2010) Investigation on solar photocatalytic degradation of various dyes in the presence of Er3+: YAlO3/ZnO–TiO2 composite. J Environ Manag 91:677–684
Hasani K, Moradi M, Mokhtari SA, Dargahi A, Vosoughi M (2021) Degradation of basic violet 16 dye by electro-activated persulfate process from aqueous solutions and toxicity assessment using microorganisms: determination of by-products, reaction kinetic and optimization using Box-Behnken design. Int J Chem Reactor Eng 19:261–275
Almasi A, Mahmoudi M, Mohammadi M, Dargahi A, Biglari H (2021) Optimizing biological treatment of petroleum industry wastewater in a facultative stabilization pond for simultaneous removal of carbon and phenol. Toxin Rev 40:189–197
Dargahi A, Ansari A, Nematollahi D, Asgari G, Shokoohi R, Samarghandi MR (2019) Parameter optimization and degradation mechanism for electrocatalytic degradation of 2, 4-diclorophenoxyacetic acid (2, 4-D) herbicide by lead dioxide electrodes. RSC Adv 9:5064–5075
Rahmani A, Salari M, Tari K, Shabanloo A, Shabanloo N, Bajalan S (2020) Enhanced degradation of furfural by heat-activated persulfate/nZVI-rGO oxidation system: Degradation pathway and improving the biodegradability of oil refinery wastewater. J Environ Chem Eng 8:104468
Dargahi A, Mohammadi M, Amirian F, Karami A, Almasi A (2017) Phenol removal from oil refinery wastewater using anaerobic stabilization pond modeling and process optimization using response surface methodology (RSM). Desalin Water Treat 87:199–208
Rahmani A, Seid-Mohammadi A, Leili M, Shabanloo A, Ansari A, Alizadeh S, Nematollahi D (2021) Electrocatalytic degradation of diuron herbicide using three-dimensional carbon felt/β-PbO2 anode as a highly porous electrode: influencing factors and degradation mechanisms. Chemosphere 276:130141
Azizi A, Dargahi A, Almasi A (2019) Biological removal of diazinon in a moving bed biofilm reactor–process optimization with central composite design. Toxin Rev 1–11
Almasi A, Dargahi A, Mohammadi M, Azizi A, Karami A, Baniamerian F, Saeidimoghadam Z (2016) Application of response surface methodology on cefixime removal from aqueous solution by ultrasonic/photooxidation. Int J Pharm Technol 8:16728–16736
Heidari M, Vosoughi M, Sadeghi H, Dargahi A, Mokhtari SA (2020) Degradation of diazinon from aqueous solutions by electro-Fenton process: effect of operating parameters, intermediate identification, degradation pathway, and optimization using response surface methodology (RSM). Sep Sci Technol 1–13
Shokoohi R, Jafari AJ, Dargahi A, Torkshavand Z (2017) Study of the efficiency of bio-filter and activated sludge (BF/AS) combined process in phenol removal from aqueous solution: determination of removing model according to response surface methodology (RSM). Desalin Water Treat 77:256–263
Alizadeh S, Sadeghi H, Vosoughi M, Dargahi A, Mokhtari SA (2020) Removal of humic acid from aqueous media using sono-persulphate process: optimization and modelling with response surface methodology (RSM). Int J Environ Anal Chem 1–15
Molla Mahmoudi M, Khaghani R, Dargahi A, Monazami Tehrani G (2020) Electrochemical degradation of diazinon from aqueous media using graphite anode: effect of parameters, mineralisation, reaction kinetic, degradation pathway and optimisation using central composite design. Int J Environ Anal Chem 1–26
Hu Y, Yuan C (2006) Low-temperature preparation of photocatalytic TiO 2 thin films on polymer substrates by direct deposition from anatase sol. Cailiao Kexue Yu Jishu (J Mater Sci Technol) 22:239–244
Samarghandi MR, Dargahi A, Rahmani A, Shabanloo A, Ansari A, Nematollahi D (2021) Application of a fluidized three-dimensional electrochemical reactor with Ti/SnO2–Sb/β-PbO2 anode and granular activated carbon particles for degradation and mineralization of 2, 4-dichlorophenol: process optimization and degradation pathway. Chemosphere 279:130640
Afshin S, Rashtbari Y, Vosough M, Dargahi A, Fazlzadeh M, Behzad A, Yousefi M (2021) Application of Box-Behnken design for optimizing parameters of hexavalent chromium removal from aqueous solutions using Fe3O4 loaded on activated carbon prepared from alga: kinetics and equilibrium study. J Water Process Eng 42:102113
Samarghandi MR, Dargahi A, Shabanloo A, Nasab HZ, Vaziri Y, Ansari A (2020) Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab J Chem 13:6847–6864
Samarghandi MR, Nemattollahi D, Asgari G, Shokoohi R, Ansari A, Dargahi A (2019) Electrochemical process for 2, 4-D herbicide removal from aqueous solutions using stainless steel 316 and graphite anodes: optimization using response surface methodology. Sep Sci Technol 54:478–493
Hasani K, Peyghami A, Moharrami A, Vosoughi M, Dargahi A (2020) The efficacy of sono-electro-Fenton process for removal of cefixime antibiotic from aqueous solutions by response surface methodology (RSM) and evaluation of toxicity of effluent by microorganisms. Arab J Chem 13:6122–6139
Dargahi A, Hasani K, Mokhtari SA, Vosoughi M, Moradi M, Vaziri Y (2021) Highly effective degradation of in a three-dimensional sono-electro-Fenton (3D/SEF) system using powder activated carbon (PAC)/Fe3O4 as magnetic particle electrode. J Environ Chem Eng 105889
Dargahi A, Nematollahi D, Asgari G, Shokoohi R, Ansari A, Samarghandi MR (2018) Electrodegradation of 2, 4-dichlorophenoxyacetic acid herbicide from aqueous solution using three-dimensional electrode reactor with G/β-PbO 2 anode: Taguchi optimization and degradation mechanism determination. RSC Adv 8:39256–39268
Simonin J-P (2016) On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem Eng J 300:254–263
Dargahi A, Samarghandi MR, Shabanloo A, Mahmoudi MM, Nasab HZ (2021) Statistical modeling of phenolic compounds adsorption onto low-cost adsorbent prepared from aloe vera leaves wastes using CCD-RSM optimization: effect of parameters, isotherm, and kinetic studies. Biomass Conver Bioref 1–5. https://doi.org/10.1007/s13399-021-01601-y
Mouni L, Belkhiri L, Bollinger J-C, Bouzaza A, Assadi A, Tirri A, Dahmoune F, Madani K, Remini H (2018) Removal of methylene blue from aqueous solutions by adsorption on Kaolin: kinetic and equilibrium studies. Appl Clay Sci 153:38–45
Seid-Mohammadi A, Asgarai G, Ghorbanian Z, Dargahi A (2020) The removal of cephalexin antibiotic in aqueous solutions by ultrasonic waves/hydrogen peroxide/nickel oxide nanoparticles (US/H2O2/NiO) hybrid process. Sep Sci Technol 55:1558–1568
Shokoohi R, Dargahi A, Gilan RA, Nasab HZ, Zeynalzadeh D, Mahmoudi MM (2019) Magnetic multi-walled carbon nanotube as effective adsorbent for ciprofloxacin (CIP) removal from aqueous solutions: isotherm and kinetics studies. Int J Chem Reactor Eng 18(2):1–15
Chung H-K, Kim W-H, Park J, Cho J, Jeong T-Y, Park P-K (2015) Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J Ind Eng Chem 28:241–246
Samarghandi M, Rahmani A, Asgari G, Ahmadidoost G, Dargahi A (2018) Photocatalytic removal of cefazolin from aqueous solution by AC prepared from mango seed+ ZnO under uv irradiation. Glob NEST J 20:399–407
Samarghandi MR, Asgari G, Shokoohi R, Dargahi A, Arabkouhsar A (2019) Removing amoxicillin antibiotic from aqueous solutions by Saccharomyces cerevisiae bioadsorbent: kinetic, thermodynamic and isotherm studies. Desalin Water Treat 152:306–315
Seidmohammadi A, Vaziri Y, Dargahi A, Nasab HZ (2021) Improved degradation of metronidazole in a heterogeneous photo-Fenton oxidation system with PAC/Fe3O4 magnetic catalyst: biodegradability, catalyst specifications, process optimization, and degradation pathway. Biomass Conver Bioref 1–7. https://doi.org/10.1007/s13399-021-01668-7
Mahmoudi MM, Nasseri S, Mahvi AH, Dargahi A, Khubestani MS, Salari M (2019) Fluoride removal from aqueous solution by acid-treated clinoptilolite: isotherm and kinetic study. Desalin Water Treat 146:333–340
Shokoohi R, Gillani RA, Mahmoudi MM, Dargahi A (2018) Investigation of the efficiency of heterogeneous Fenton-like process using modified magnetic nanoparticles with sodium alginate in removing Bisphenol A from aquatic environments: kinetic studies. Desalin Water Treat 101:185–192
Rahmani AR, Salari M, Shabanloo A, Shabanloo N, Bajalan S, Vaziri Y (2020) Sono-catalytic activation of persulfate by nZVI-reduced graphene oxide for degradation of nonylphenol in aqueous solution: process optimization, synergistic effect and degradation pathway. J Environ Chem Eng 8:104202
Shokoohi R, Bajalan S, Salari M, Shabanloo A (2019) Thermochemical degradation of furfural by sulfate radicals in aqueous solution: optimization and synergistic effect studies. Environ Sci Pollut Res 26:8914–8927
Dargahi A, Shokoohi R, Asgari G, Ansari A, Nematollahi D, Samarghandi MR (2021) Moving-bed biofilm reactor combined with three-dimensional electrochemical pretreatment (MBBR–3DE) for 2, 4-D herbicide treatment: application for real wastewater, improvement of biodegradability. RSC Adv 11:9608–9620
Powers M, Yracheta J, Harvey D, O’Leary M, Best LG, Bear AB, MacDonald L, Susan J, Hasan K, Thomas E (2019) Arsenic in groundwater in private wells in rural North Dakota and South Dakota: water quality assessment for an intervention trial. Environ Res 168:41–47
Tsimas ES, Tyrovola K, Xekoukoulotakis NP, Nikolaidis NP, Diamadopoulos E, Mantzavinos D (2009) Simultaneous photocatalytic oxidation of As (III) and humic acid in aqueous TiO2 suspensions. J Hazard Mater 169:376–385
Altundoğan HS, Altundoğan S, Tümen F, Bildik M (2000) Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag 20:761–767
Funding
The current study was financially supported by Tehran North Branch, Islamic Azad University, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Nastuna Ghanbari Sagharloo: conceptualization, methodology, validation, formal analysis, investigation, resources, supervision, funding acquisition. Mohammad rabani: methodology; validation; resources; writing, original draft; writing, review and editing. Lida salimi: analyses, writing and text revision. Hossein Ghafourian and S.M.T Sadatipour: methodology, validation, formal analysis, investigation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sagharloo, N.G., Rabani, M., Salimi, L. et al. Immobilized ZnO/TiO2 activated carbon (I ZnO/TiO2 AC) to removal of arsenic from aqueous environments: optimization using response surface methodology and kinetic studies. Biomass Conv. Bioref. 13, 10483–10494 (2023). https://doi.org/10.1007/s13399-021-01741-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01741-1