Abstract
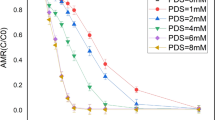
In this study, thermochemical degradation of furfural by sulfate radical has been investigated to find the best-operating conditions. For this purpose, the response surface methodology (RSM) based on central composite design (CCD) was applied to optimize the five independent variables of thermally activated persulfate (TAP)/nZVI oxidation process including pH, PS concentration, furfural concentration, nZVI dosage, and heat. The ANOVA results (“P > F value” < 0.0001 and \( {\mathrm{R}}_{\mathrm{adj}}^2 \) = 0.9701) showed the obtained quadratic model is acceptable to predict furfural removal. Based on the reduced quadratic model PS concentration, nZVI dosage, and heat revealed the positive effects on removal efficiency, while pH and furfural concentration had a negative effect. Accordingly, 98.4% of furfural could be removed within 60 min of reaction under the optimum conditions: pH 5.26, PS concentration of 20.52 mM, furfural concentration of 84.32 mg/L, nZVI dosage of 1.15 mg/L, and a temperature of 79 °C. In such circumstances, the furfural removal efficiency for TAP, PS/nZVI, PS, and nZVI was 94.5, 9, 3, and 2%, respectively. Therefore, based on the synergy index (SI) values, the combination of PS, nZVI, and heat can lead to a synergistic effect in the performance of the thermochemical process.










Similar content being viewed by others
References
Abdessalem AK, Oturan N, Bellakhal N, Dachraoui M, Oturan MA (2008) Experimental design methodology applied to electro-Fenton treatment for degradation of herbicide chlortoluron. Appl Catal B Environ 78:334–341
Anupam K, Dutta S, Bhattacharjee C, Datta S (2011) Adsorptive removal of chromium (VI) from aqueous solution over powdered activated carbon: optimisation through response surface methodology. Chem Eng J 173:135–143
Asfaram A, Ghaedi M, Ghezelbash GR, Dil EA, Tyagi I, Agarwal S, Gupta VK (2016) Biosorption of malachite green by novel biosorbent Yarrowia lipolytica isf7: application of response surface methodology. J Mol Liq 214:249–258
Babuponnusami A, Muthukumar K (2012) Removal of phenol by heterogenous photo electro Fenton-like process using nano-zero valent iron. Sep Purif Technol 98:130–135
Borghei SM, Hosseini SN (2008) Comparison of furfural degradation by different photooxidation methods. Chem Eng J 139:482–488
Cai J, Zhou M, Yang W, Pan Y, Lu X, Serrano KG (2018) Degradation and mechanism of 2,4-dichlorophenoxyacetic acid (2,4-D) by thermally activated persulfate oxidation. Chemosphere 212:784–793
Chakma S, Praneeth S, Moholkar VS (2017) Mechanistic investigations in sono-hybrid (ultrasound/Fe2+/UVC) techniques of persulfate activation for degradation of Azorubine. Ultrason Sonochem 38:652–663
Chen X, Murugananthan M, Zhang Y (2016) Degradation of p-Nitrophenol by thermally activated persulfate in soil system. Chem Eng J 283:1357–1365
Cho I-H, Zoh K-D (2007) Photocatalytic degradation of azo dye (reactive red 120) in TiO2/UV system: optimization and modeling using a response surface methodology (RSM) based on the central composite design. Dyes Pigments 75:533–543
Cuevas M, Quero SM, Hodaifa G, López AJM, Sánchez S (2014) Furfural removal from liquid effluents by adsorption onto commercial activated carbon in a batch heterogeneous reactor. Ecol Eng 68:241–250
Deng J, Shao Y, Gao N, Deng Y, Zhou S, Hu X (2013) Thermally activated persulfate (TAP) oxidation of antiepileptic drug carbamazepine in water. Chem Eng J 228:765–771
Dong H, He Q, Zeng G, Tang L, Zhang L, **e Y, Zeng Y, Zhao F (2017) Degradation of trichloroethene by nanoscale zero-valent iron (nZVI) and nZVI activated persulfate in the absence and presence of EDTA. Chem Eng J 316:410–418
Durán A, Monteagudo JM, Expósito AJ, Monsalve V (2016) Modeling the sonophoto-degradation/mineralization of carbamazepine in aqueous solution. Chem Eng J 284:503–512
Eslami A, Asadi A, Meserghani M, Bahrami H (2016) Optimization of sonochemical degradation of amoxicillin by sulfate radicals in aqueous solution using response surface methodology (RSM). J Mol Liq 222:739–744
Faramarzpour M, Vossoughi M, Borghei M (2009) Photocatalytic degradation of furfural by titania nanoparticles in a floating-bed photoreactor. Chem Eng J 146:79–85
Ferkous H, Merouani S, Hamdaoui O, Pétrier C (2017) Persulfate-enhanced sonochemical degradation of naphthol blue black in water: evidence of sulfate radical formation. Ultrason Sonochem 34:580–587
Frontistis Z, Antonopoulou M, Konstantinou I, Mantzavinos D (2017) Degradation of ethyl paraben by heat-activated persulfate oxidation: statistical evaluation of operating factors and transformation pathways. Environ Sci Pollut Res 24:1073–1084
Gao Y-Q, Gao N-Y, Deng Y, Yin D-Q, Zhang Y-S, Rong W-L, Zhou S-D (2015) Heat-activated persulfate oxidation of sulfamethoxazole in water. Desalin Water Treat 56:2225–2233
Gao Y-Q, Gao N-Y, Wang W, Kang S-F, Xu J-H, **ang H-M, Yin D-Q (2018) Ultrasound-assisted heterogeneous activation of persulfate by nano zero-valent iron (nZVI) for the propranolol degradation in water. Ultrason Sonochem 49:33–40
George C, Rassy HE, Chovelon JM (2001) Reactivity of selected volatile organic compounds (VOCs) toward the sulfate radical (SO4−). In J Chem Kinet 33:539–547
Ghafari S, Aziz HA, Isa MH, Zinatizadeh AA (2009) Application of response surface methodology (RSM) to optimize coagulation–flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 163:650–656
Ghauch A, Tuqan AM, Kibbi N (2012) Ibuprofen removal by heated persulfate in aqueous solution: a kinetics study. Chem Eng J 197:483–492
Hazime R, Nguyen QH, Ferronato C, Huynh TKX, Jaber F, Chovelon JM (2013) Optimization of imazalil removal in the system UV/TiO2/K2S2O8 using a response surface methodology (RSM). Appl Catal B Environ 132:519–526
He Y, Pei M, Du Y, Yu F, Wang L, Guo W (2014) Synthesis, characterization and application of chitosan coated Fe3O4 particles as an adsorbent for the removal of furfural from aqueous solution. RSC Adv 4:30352–30357
Hu P, Long M (2016) Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl Catal B Environ 181:103–117
Huang K-C, Couttenye RA, Hoag GE (2002) Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 49:413–420
Hussain I, Zhang Y, Huang S, Du X (2012) Degradation of p-chloroaniline by persulfate activated with zero-valent iron. Chem Eng J 203:269–276
Jafarinejad S (2017) Activated sludge combined with powdered activated carbon (PACT process) for the petroleum industry wastewater treatment: a review. Chem Int 3:268–277
Ji Y, Fan Y, Liu K, Kong D, Lu J (2015) Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res 87:1–9
Ji Y, **e W, Fan Y, Shi Y, Kong D, Lu J (2016) Degradation of trimethoprim by thermo-activated persulfate oxidation: reaction kinetics and transformation mechanisms. Chem Eng J 286:16–24
Khan S, He X, Khan JA, Khan HM, Boccelli DL, Dionysiou DD (2017) Kinetics and mechanism of sulfate radical-and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem Eng J 318:135–142
Lau TK, Chu W, Graham NJ (2007) The aqueous degradation of butylated hydroxyanisole by UV/S2O82-: study of reaction mechanisms via dimerization and mineralization. Environ Sci Technol 41:613–619
Li R, Cai M, Liu H, Liu G, Lv W (2018) Thermo-activated peroxydisulfate oxidation of indomethacin: kinetics study and influences of co-existing substances. Chemosphere 212:1067–1075
Liang C, Su H-W (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562
Lin Y-T, Liang C, Chen J-H (2011) Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chemosphere 82:1168–1172
Liu A, Liu J, W-x Z (2015) Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water. Chemosphere 119:1068–1074
Liu L, Lin S, Zhang W, Farooq U, Shen G, Hu S (2018) Kinetic and mechanistic investigations of the degradation of sulfachloropyridazine in heat-activated persulfate oxidation process. Chem Eng J 346:515–524
Lucas S, Cocero MJ, Zetzl C, Brunner G (2004) Adsorption isotherms for ethylacetate and furfural on activated carbon from supercritical carbon dioxide. Fluid Phase Equilib 219:171–179
Moghaddam SS, Moghaddam MA, Arami M (2010) Coagulation/flocculation process for dye removal using sludge from water treatment plant: optimization through response surface methodology. J Hazard Mater 175:651–657
Moradi M, Ghanbari F (2014) Application of response surface method for coagulation process in leachate treatment as pretreatment for Fenton process: biodegradability improvement. J Water Process Eng 4:67–73
Moradi M, Ghanbari F, Manshouri M, Angali KA (2016) Photocatalytic degradation of azo dye using nano-ZrO2/UV/persulfate: response surface modeling and optimization. Korean J Chem Eng 33:539–546
Nezamzadeh-Ejhieh A, Moeinirad S (2011) Heterogeneous photocatalytic degradation of furfural using NiS-clinoptilolite zeolite. Desalination 273:248–257
Nie M, Yang Y, Zhang Z, Yan C, Wang X, Li H, Dong W (2014) Degradation of chloramphenicol by thermally activated persulfate in aqueous solution. Chem Eng J 246:373–382
Norzaee S, Taghavi M, Djahed B, Kord Mostafapour F (2018) Degradation of penicillin G by heat activated persulfate in aqueous solution. J Environ Manag 215:316–323
Oh S-Y, Kim H-W, Park J-M, Park H-S, Yoon C (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe 2+, and zero-valent iron. J Hazard Mater 168:346–351
Olmez-Hanci T, Arslan-Alaton I (2013) Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem Eng J 224:10–16
Priya, Kaith BS, Shanker U, Gupta B, Bhatia JK (2018) RSM-CCD optimized in-air synthesis of photocatalytic nanocomposite: application in removal-degradation of toxic brilliant blue. React Funct Polym 131:107–122
Purkait MK, Maiti A, Dasgupta S, De S (2007) Removal of congo red using activated carbon and its regeneration. J Hazard Mater 145:287–295
Qian Y, Xue G, Chen J, Luo J, Zhou X, Gao P, Wang Q (2018) Oxidation of cefalexin by thermally activated persulfate: kinetics, products, and antibacterial activity change. J Hazard Mater 354:153–160
Rahmani AR, Rezaeivahidian H, Almasi M, Shabanlo A, Almasi H (2016) A comparative study on the removal of phenol from aqueous solutions by electro–Fenton and electro–persulfate processes using iron electrodes. Res Chem Intermed 42:1441–1450
Rahmani AR, Shabanloo A, Fazlzadeh M, Poureshgh Y, Rezaeivahidian H (2017) Degradation of acid blue 113 in aqueous solutions by the electrochemical advanced oxidation in the presence of persulfate. Desalin Water Treat 59:202–209
Rani SK, Easwaramoorthy D, Bilal IM, Palanichamy M (2009) Studies on Mn (II)-catalyzed oxidation of α-amino acids by peroxomonosulphate in alkaline medium-deamination and decarboxylation: a kinetic approach. Appl Catal A Gen 369:1–7
Sahu AK, Mall ID, Srivastava VC (2007) Studies on the adsorption of furfural from aqueous solution onto low-cost bagasse fly ash. Chem Eng Commun 195:316–335
Sahu AK, Srivastava VC, Mall ID, Lataye DH (2008) Adsorption of furfural from aqueous solution onto activated carbon: kinetic, equilibrium and thermodynamic study. Sep Sci Technol 43:1239–1259
Seid-Mohammadi A, Shabanloo A, Fazlzadeh M, Poureshgh Y (2017) Degradation of acid blue 113 by US/H2O2/Fe2+ and US/S2O8 2–/Fe2+ processes from aqueous solutions. Desalin Water Treat 78:273–280
Sharma J, Anand P, Pruthi V, Chaddha AS, Bhatia J, Kaith B (2017) RSM-CCD optimized adsorbent for the sequestration of carcinogenic rhodamine-B: kinetics and equilibrium studies. Mater Chem Phys 196:270–283
Singh S, Srivastava VC, Mall ID (2009) Fixed-bed study for adsorptive removal of furfural by activated carbon. Colloids Surf A Physicochem Eng Asp 332:50–56
Song H, Yan L, Jiang J, Ma J, Zhang Z, Zhang J, Liu P, Yang T (2018) Electrochemical activation of persulfates at BDD anode: radical or nonradical oxidation? Water Res 128:393–401
Tan C, Gao N, Deng Y, Li L, Deng J, Zhou S (2015) Kinetic oxidation of antipyrine in heat-activated persulfate. Desalin Water Treat 53:263–271
Veisi F, Zazouli MA, Ebrahimzadeh MA, Charati JY, Dezfoli AS (2016) Photocatalytic degradation of furfural in aqueous solution by N-doped titanium dioxide nanoparticles. Environ Sci Pollut Res 23:21846–21860
Vilardi G, Sebastiani D, Miliziano S, Verdone N, Di Palma L (2018) Heterogeneous nZVI-induced Fenton oxidation process to enhance biodegradability of excavation by-products. Chem Eng J 335:309–320
Wang X, Wang L, Li J, Qiu J, Cai C, Zhang H (2014) Degradation of acid orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Sep Purif Technol 122:41–46
Wang T, Meng Y, Qin Y, Feng W, Wang C (2018a) Removal of furfural and HMF from monosaccharides by nanofiltration and reverse osmosis membranes. J Energy Inst 91:473–480
Wang Z, Shao Y, Gao N, Lu X, An N (2018b) Degradation kinetic of phthalate esters and the formation of brominated byproducts in heat-activated persulfate system. Chem Eng J
Wei L-L, Chen W-M, Li Q-B, Gu Z-P, Zhang A-P (2018) Treatment of dinitrodiazophenol industrial wastewater in heat-activated persulfate system. RSC Adv 8:20603–20611
Wirtz RA, Dague RR (1993) Anaerobic treatment of a furfural-production wastewater. Waste Manag 13:309–315
Yehia FZ, Eshaq G, Rabie AM, Mady AH, ElMetwally AE (2015) Phenol degradation by advanced Fenton process in combination with ultrasonic irradiation. Egypt J Pet 24:13–18
Zarei AR, Rezaeivahidian H, Soleymani AR (2015) Investigation on removal of p-nitrophenol using a hybridized photo-thermal activated persulfate process: central composite design modeling. Process Saf Environ Prot 98:109–115
Zeitsch KJ (2000) The chemistry and technology of furfural and its many by-products. Sugar series, vol 13. Elsevier, Amsterdam
Zhang D, Ong YL, Li Z, Wu JC (2013) Biological detoxification of furfural and 5-hydroxyl methyl furfural in hydrolysate of oil palm empty fruit bunch by Enterobacter sp. FDS8. Biochem Eng J 72:77–82
Zhao L, Hou H, Fujii A, Hosomi M, Li F (2014) Degradation of 1,4-dioxane in water with heat- and Fe2+-activated persulfate oxidation. Environ Sci Pollut Res 21:7457–7465
Zhou R, Li T, Su Y, Ma T, Zhang L, Ren H (2018) Oxidative removal of metronidazole from aqueous solution by thermally activated persulfate process: kinetics and mechanisms. Environ Sci Pollut Res 25:2466–2475
Acknowledgments
The authors appreciate the supports from the Hamadan University of Medical Sciences of Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ioannis A. Katsoyiannis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shokoohi, R., Bajalan, S., Salari, M. et al. Thermochemical degradation of furfural by sulfate radicals in aqueous solution: optimization and synergistic effect studies. Environ Sci Pollut Res 26, 8914–8927 (2019). https://doi.org/10.1007/s11356-019-04382-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04382-0