Abstract
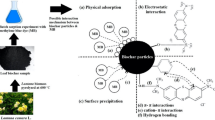
Torrefaction, as a pretreatment of biomass coupled with pyrolysis process, has gathered significant attention in recent years to obtain higher quality bio-oil. Being an energy-intensive process, the application of byproducts such as biochar from the integrated process might offset the extra energy provided during the torrefaction process. With this hypothesis, present work aimed to investigate the performance of biochar (BC) obtained from pyrolysis of native (BC-raw) and torrefied biomass (BC-torrefied) towards the synthetic wastewater treatment containing methylene blue (MB) dye. Both biochars were characterized by their physicochemical properties. The impact of time, dose of adsorbent, pH, concentration of MB, and temperature were examined during batch adsorption process. Results showed that BC-torrefied (103.47 m2/g) has higher Brunauer–Emmett–Teller (BET) surface area than BC-raw (80.40 m2/g). Both biochars behave in similar fashion toward MB removal; however, BC-torrefied had greater adsorption capacity (158.13 mg/g from Sips isotherm at 50 mg/L MB concentration) because of higher BET surface area, pore volume, and complexation on surface as compared to BC-raw (85.68 mg/g from Sips isotherm at 50 mg/L MB concentration). The experimental data were in good agreement with Sips isotherm and pseudo-second-order kinetic model for both biochars. The MB adsorption was unprompted and endothermic. Further, it was observed that hydrogen bonding, electrostatic force of attraction, ion exchange, surface complexation, and \(\pi\)-\(\pi\) interaction between MB dye and adsorbent were primarily accountable for adsorption process. Thus, BC-torrefied could be novel adsorbent for water treatment, and its application will facilitate the integrated torrefaction-pyrolysis process.











Similar content being viewed by others
References
Gupta GK, Mondal MK (2020) Mechanism of Cr(VI) uptake onto sagwan sawdust derived biochar and statistical optimization via response surface methodology. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-020-01082-5
Sangon S, Hunt AJ, Attard TM, Mengchang P, Ngernyen Y, Supanchaiyamat N (2018) Valorisation of waste rice straw for the production of highly effective carbon based adsorbents for dyes removal. J Clean Prod 172:1128–1139
Lyu H, Gao B, He F, Zimmerman AR, Ding C, Tang J (2018) Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem Eng J 335:110–119
Zhai S, Li M, Wang D, Zhang L, Yang Y, Fu S (2019) In situ loading metal oxide particles on bio-chars: reusable materials for efficient removal of methylene blue from wastewater. J Clean Prod 220:460–474
Mahmoud DK, Salleh MAM, Karim WAWA, Idris A, Abidin ZZ (2012) Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 181:449–457
Liu S, Li J, Xu S, Wang M, Zhang Y, Xue X (2019) A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour Technol 282:48–55
Li Y, Zhang Y, Wang G, Li S, Han R, Wei W (2018) Reed biochar supported hydroxyapatite nanocomposite: characterization and reactivity for methylene blue removal from aqueous media. J Mol Liq 263:53–63
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Jouali A, Salhi A, Aguedach A, Aarfane A, Ghazzaf H, Lhadi E (2019) Photo-catalytic degradation of methylene blue and reactive blue 21 dyes in dynamic mode using TiO2 particles immobilized on cellulosic fibers. J Photochem Photobio A: Chem 383:112013
Parakala S, Moulik S, Sridhar S (2019) Effective separation of methylene blue dye from aqueous solutions by integration of micellar enhanced ultrafiltration with vacuum membrane distillation. Chem Eng J 375:122015
Duan X, Wu P, Pi K, Zhang H, Liu D, Gerson AR (2018) Application of modified electrocoagulation for efficient color removal from synthetic Methylene blue wastewater. Int J Electrochem Sci 13:5575–5588
Fan L, Zhou Y, Yang W, Chen G, Yang F (2008) Electrochemical degradation of aqueous solution of Amaranth azo dye on ACF under potentiostatic model. Dyes Pigm 76:440–446
Wanassi B, Hariz IB, Ghimbeu CM, Vaulot C, Hassen MB, Jeguirim M (2017) Carbonaceous adsorbents derived from textile cotton waste for the removal of Alizarin S dye from aqueous effluent: kinetic and equilibrium studies. Env Sci Pollution Res 24:10041–10055
Benhouria A, Islam MA, Zaghouane-Boudiaf H, Boutahala M, Hameed B (2015) Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chem Eng J 270:621–630
Yang Q, Ren S, Zhao Q, Lu R, Hang C, Chen Z (2018) Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem Eng J 333:49–57
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant soil 300:9–20
Shafie ST, Salleh MM, Hang LL, Rahman M, Ghani W (2012) Effect of pyrolysis temperature on the biochar nutrient and water retention capacity. J Purity Utility Reaction Env 1:293–307
Liesch AM, Weyers SL, Gaskin JW, Das K (2010) Impact of two different biochars on earthworm growth and survival. Annals Env Sci 4:1–9
Zhang P, O’Connor D, Wang Y, Jiang L, **a T, Wang L (2020) A green biochar/iron oxide composite for methylene blue removal. J Hazard Mater 384:121286
Trakal L, Šigut R, Šillerová H, Faturíková D, Komárek M (2014) Copper removal from aqueous solution using biochar: Effect of chemical activation. Arab J Chem 7:43–52
Uchimiya M, Lima IM, Thomas Klasson K, Chang S, Wartelle LH, Rodgers JE (2010) Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J Agri Food Chem 58:5538–5544
Singh S, Chakraborty JP, Mondal MK (2020) Torrefaction of woody biomass (Acacia nilotica): investigation of fuel and flow properties to study its suitability as a good quality solid fuel. Renew Energy 153:711–724
Singh S, Chakraborty JP, Mondal MK (2019) Optimization of process parameters for torrefaction of Acacia nilotica using response surface methodology and characteristics of torrefied biomass as upgraded fuel. Energy 186:115865
He Q, Ding L, Gong Y, Li W, Wei J, Yu G (2019) Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis. Bioresour Technol 280:104–111
Cardona S, Gallego LJ, Valencia V, Martínez E, Rios LA (2019) Torrefaction of eucalyptus-tree residues: a new method for energy and mass balances of the process with the best torrefaction conditions. Sustain Energy Technol Asses 31:17–24
Singh S, Chakraborty JP, Mondal MK (2020) Torrefaction of Acacia nilotica: oxygen distribution and carbon densification mechanism based on in-depth analyses of solid, liquid, and gaseous products. Energy Fuels 34:12586–12597
Dai L, Wang Y, Liu Y, Ruan R, He C, Yu Z (2019) Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: a state-of-the-art review. Renew Sustain Energy Rev 107:20–36
Ji B, Wang J, Song H, Chen W (2019) Removal of methylene blue from aqueous solutions using biochar derived from a fallen leaf by slow pyrolysis: Behavior and mechanism. J Environ Chem Eng 7:103036
Chen S, Qin C, Wang T, Chen F, Li X, Hou H (2019) Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J Mol Liq 285:62–74
Dawood S, Sen TK, Phan C (2016) Adsorption removal of Methylene Blue (MB) dye from aqueous solution by bio-char prepared from Eucalyptus sheathiana bark: kinetic, equilibrium, mechanism, thermodynamic and process design. Desalin Wat Treat 57:28964–28980
Yu KL, Lee XJ, Ong HC, Chen W-H, Chang J-S, Lin C-S (2021) Adsorptive removal of cationic methylene blue and anionic Congo red dyes using wet-torrefied microalgal biochar: Equilibrium, kinetic and mechanism modeling. Environ Pollution 272:115986
Zhu Y, Yi B, Yuan Q, Wu Y, Wang M, Yan S (2018) Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv 8(36):19917–19929
Doddapaneni TRKC, Jain R, Praveenkumar R, Rintala J, Romar H, Konttinen J (2018) Adsorption of furfural from torrefaction condensate using torrefied biomass. Chem Eng J 334:558–568
Salapa I, Haralampous P, Giakoumakis G, Nazos A, Sidiras D (2018) Torrefaction of barley straw for the co-production of energy and adsorbent materials. Proceedings of the 4th World Congress on Mechanical, Chemical, and Material Engineering (MCM’18), Madrid, Spain 16–8
Gupta TB, Lataye DH (2019) Removal of crystal violet and methylene blue dyes using Acacia Nilotica sawdust activated carbon. IJCT 26(1):52–68
Garg R, Anand N, Kumar D (2016) Pyrolysis of babool seeds (Acacia nilotica) in a fixed bed reactor and bio-oil characterization. Renew Energy 96:167–171
Singh S, Chakraborty JP, Mondal MK (2020) Pyrolysis of torrefied biomass: optimization of process parameters using response surface methodology, characterization, and comparison of properties of pyrolysis oil from raw biomass. J Clean Prod 272:122517
Liu S, Ni C, Su H, Liu H, Chen R, Li P (2016) Exploring the critical dependence of the adsorption of various dyes on the degradation rate using a ferrihydrite surface under visible light. RSC adv 6:30840–30845
Ronix A, Pezoti O, Souza LS, Souza IP, Bedin KC, Souza PS (2017) Hydrothermal carbonization of coffee husk: optimization of experimental parameters and adsorption of methylene blue dye. J Environ Chem Eng 5:4841–4849
Fan S, Wang Y, Wang Z, Tang J, Tang J, Li X (2017) Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: adsorption kinetics, equilibrium, thermodynamics and mechanism. J Environ Chem Eng 5:601–611
Zubair M, Manzar MS, Mu’azu ND, Anil I, Blaisi NI, Al-Harthi MA (2020) Functionalized MgAl-layered hydroxide intercalated date-palm biochar for enhanced uptake of cationic dye: kinetics, isotherm and thermodynamic studies. Appl Clay Sci 190:105587
Yao X, Ji L, Guo J, Ge S, Lu W, Cai L (2020) Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption. Bioresour Technol 302:122842
Yao X, Ji L, Guo J, Ge S, Lu W, Chen Y (2020) An abundant porous biochar material derived from wakame (Undaria pinnatifida) with high adsorption performance for three organic dyes. Bioresour Technol 318:124082
Huang Y, Yin X, Wu C, Wang C, **e J, Zhou Z (2009) Effects of metal catalysts on CO2 gasification reactivity of biomass char. Biotechnol adv 27:568–572
Mohan D, Abhishek K, Sarswat A, Patel M, Singh P, Pittman CU (2018) Biochar production and applications in soil fertility and carbon sequestration – a sustainable solution to crop-residue burning in India. RSC Adv 8:508–520
Liu Y, Zhao X, Li J, Ma D, Han R (2012) Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalin Wat Treat 46:115–123
Alinejad-Mir A, Amooey AA, Ghasemi S (2018) Adsorption of direct yellow 12 from aqueous solutions by an iron oxide-gelatin nanoadsorbent; kinetic, isotherm and mechanism analysis. J Clean Prod 170:570–580
Banerjee S, Chattopadhyaya M (2017) Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab J Chem 10:S1629–S1638
Ye J, Cong X, Zhang P, Hoffmann E, Zeng G, Wu Y (2015) Phosphate adsorption onto granular-acid-activated-neutralized red mud: parameter optimization, kinetics, isotherms, and mechanism analysis. Water Air Soil Pollution 226:306
Saini J, Garg V, Gupta R (2018) Removal of Methylene Blue from aqueous solution by Fe3O4@ Ag/SiO2 nanospheres: synthesis, characterization and adsorption performance. J Mol Liq 250:413–422
Huang T, Yan M, He K, Huang Z, Zeng G, Chen A (2019) Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J Colloid Interface Sci 543:43–51
Wang H, **e R, Zhang J, Zhao J (2018) Preparation and characterization of distillers’ grain based activated carbon as low cost methylene blue adsorbent: mass transfer and equilibrium modeling. Adv Powder Technol 29:27–35
Prajapati AK, Mondal MK (2019) Hazardous As(III) removal using nanoporous activated carbon of waste garlic stem as adsorbent: kinetic and mass transfer mechanisms. Korean J Chem Eng 36:1900–1914
Vargas AM, Cazetta AL, Kunita MH, Silva TL, Almeida VC (2011) Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chem Eng J 168:722–730
Shahryari Z, Goharrizi AS, Azadi M (2010) Experimental study of methylene blue adsorption from aqueous solutions onto carbon nano tubes. Int J Water Res Env Eng 2:016–028
Liu J, Wang Y, Fang Y, Mwamulima T, Song S, Peng C (2018) Removal of crystal violet and methylene blue from aqueous solutions using the fly ash-based adsorbent material-supported zero-valent iron. J Mol Liq 250:468–476
Fan S, Tang J, Wang Y, Li H, Zhang H, Tang J (2016) Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: kinetics, isotherm, thermodynamic and mechanism. J Mol Liq 220:432–441
Sun L, Wan S, Luo W (2013) Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: characterization, equilibrium, and kinetic studies. Bioresour Technol 140:406–413
** Z, Wang X, Sun Y, Ai Y, Wang X (2015) Adsorption of 4-n-nonylphenol and bisphenol-A on magnetic reduced graphene oxides: a combined experimental and theoretical studies. Environ Sci Technol 49:9168–9175
Shi L, Zhang G, Wei D, Yan T, Xue X, Shi S (2014) Preparation and utilization of anaerobic granular sludge-based biochar for the adsorption of methylene blue from aqueous solutions. J Mol Liq 198:334–340
Liu Q, Li Y, Chen H, Lu J, Yu G, Möslang M (2020) Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J Hazard Mater 382:121040
Ding Z, Hu X, Zimmerman AR, Gao B (2014) Sorption and cosorption of lead (II) and methylene blue on chemically modified biomass. Bioresour Technol 167:569–573
Wang Y, Zhang Y, Li S, Zhong W, Wei W (2018) Enhanced methylene blue adsorption onto activated reed-derived biochar by tannic acid. J Mol Liq 268:658–666
Huang W, Chen J, Zhang J (2018) Adsorption characteristics of methylene blue by biochar prepared using sheep, rabbit and pig manure. Env Sci Pollution Res 25:29256–29266
Guo D, Li Y, Cui B, Hu M, Luo S, Ji B (2020) Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. J Clean Prod 267:121903
Li L, Yang M, Lu Q, Zhu W, Ma H, Dai L (2019) Oxygen-rich biochar from torrefaction: a versatile adsorbent for water pollution control. Bioresour Technol 294:122142
Funding
The authors acknowledge the funding from Science and Engineering Research Board (SERB), New Delhi, India, through fund no. SR/FTP/ETA-56/2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, S., Prajapati, A.K., Chakraborty, J.P. et al. Adsorption potential of biochar obtained from pyrolysis of raw and torrefied Acacia nilotica towards removal of methylene blue dye from synthetic wastewater. Biomass Conv. Bioref. 13, 6083–6104 (2023). https://doi.org/10.1007/s13399-021-01645-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01645-0