Abstract
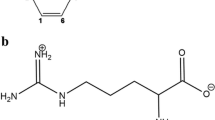
The aim of the present investigation was to enhance the solubility and dissolution of atorvastatin calcium (ATV), a poorly water-soluble drug with larch polysaccharide arabinogalactan (AG) and disodium glycyrrhizate (Na2GA) as carriers of drug delivery systems for improving its bioavailability. The interactions of ATV with AG or Na2GA were investigated by DSC, XRD, SEM, and NMR techniques. The molecular weights of supramolecular systems—inclusion complexes and micelles—which are the hosts for ATV molecules were measured. On the other hand, the rapid storage assay (+ 40 °C for 3 months) showed that the chemical stability of ATV/AG and ATV/Na2GA complexes had been enhanced compared with pure ATV. In vitro drug release showed a significant increase in ATV’s dissolution rate after formation of a complex with Na2GA or AG. Pharmacokinetic tests in vivo on laboratory animals showed a significant increase in ATV’s bioavailability after its introduction as a complex with Na2GA or AG. Moreover, ATV/AG and ATV/Na2GA complexes showed a more prominent decrease of total cholesterol (TC) level compared to net ATV. Therefore, the novel mechanochemically synthesized complexes of ATV with AG or Na2GA as drug delivery systems might be potential and promising candidates for hypercholesterolemia treatment and deserved further researches.









Similar content being viewed by others
Abbreviations
- AG:
-
Arabinogalactan
- API:
-
Active pharmaceutical ingredient
- ATV:
-
Atorvastatin calcium
- DSC:
-
Differential scanning calorimetry
- GA:
-
Glycyrrhizic acid
- HMG-CoA:
-
3-Hydroxy-3-methyl-glutaryl-coenzyme A
- Na2GA:
-
Disodium glycyrrhizate
- PM:
-
Physical mixture
- PXRD:
-
Powder X-ray diffractometry
- SEM:
-
Scanning electron microscopy
- TC:
-
Total cholesterol
References
Herron CE, Brueckner CC, Chism JP, Kemp DC, Prescott JS, Smith GA, et al. Toxicokinetics and toxicity of atorvastatin in dogs. Toxicol Appl Pharmacol. 2015;289:117–23.
Kim MS, ** SJ, Kim JS, Park HJ, Song HS, Neubert RHH, et al. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur J Pharm Biopharm. 2008;69:454–65.
Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmcol Ther. 1999;84:413–28.
Macdonald JS, Halleck MM. The toxicology of HMG-CoA reductase inhibitors: prediction of human risk. Toxicol Pathol. 2004;32(Suppl 2):26–41.
Pandit AP, Chavan TT, Khandelwal KR. Enhancement of solubility, dissolution rate and bioavailability of atorvastatin using solid lipid: in vitro and in vivo characterization. J Pharm Investig. 2015;45:503–13.
Anwar M, Warsi MH, Mallick N, Akhter S, Gahoi S, Jain GK, et al. Enhanced bioavailability of nano-sized chitosan-atorvastatin conjugate after oral administration to rats. Eur J Pharm Sci. 2011;44:241–9.
Hussein AK, Ibrahim MA, Amin MA, Ahmed OAA, Afouna MI. Improved in vitro dissolution parameters and in vivo hypolipidemic efficiency of atorvastatin calcium through the formation of hydrophilic inclusion complex with cyclodextrins. Drug Dev Res. 2011;72:379–90.
Lv HX, Zhang ZH, Jiang H, Waddad AY, Zhou JP. Preparation, physicochemical characteristics and bioavailability studies of an atorvastatin hydroxypropyl-beta-cyclodextrin complex. Pharmazie. 2012;67:46–53.
Palanisamy M, James A, Khanam J. Atorvastatin-cyclodextrin systems: physiochemical and biopharmaceutical evaluation. J Drug Delivery Sci Technol. 2016;31:41–52.
Kim MS, Kim JS, Cho W, Park HJ, Hwang SJ. Oral absorption of atorvastatin solid dispersion based on cellulose or pyrrolidone derivative polymers. Int J Biol Macromol. 2013;59:138–42.
Jahangiri A, Barzegar-Jalali M, Garjani A, Javadzadeh Y, Hamishehkar H, Afroozian A, et al. Pharmacological and histological examination of atorvastatin-PVP K30 solid dispersions. Powder Technol. 2015;286:538–45.
Jahangiri A, Barzegar-Jalali M, Garjani A, Javadzadeh Y, Hamishehkar H, Asadpour-Zeynal K, et al. Evaluation of physicochemical properties and in vivo efficiency of atorvastatin calcium/ezetimibe solid dispersions. Eur J Pharm Sci. 2016;82:21–30.
Prabhu P, Patravale V. Dissolution enhancement of atorvastatin calcium by cogrinding technique. Drug Deliv Transl Res. 2016;6:380–91.
Kim JS, Kim MS, Park HJ, ** SJ, Lee S, Hwang SJ. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int J Pharm. 2008;359:211–9.
Gowda DV, Bathool A, Khan MS, Shivakumar HG. Development and characterization of atorvastatin calcium loaded chitosan nanoparticles for sustain drug delivery. Adv Mater Lett. 2012;3:466–70.
Li ZB, Tao WH, Zhang D, Wu CN, Song BB, Wang S, et al. The studies of PLGA nanoparticles loading atorvastatin calcium for oral administration in vitro, and in vivo. Asian J Pharm Sci. 2017;12:285–91.
Kobayashi M, Hattori Y, Sasaki T, Otsuka M. Effect of ball milling on the physicochemical properties of atorvastatin calcium sesquihydrate: the dissolution kinetic behaviours of milled amorphous solids. J Pharm Pharmacol. 2017;69:15–22.
Khan F, Islam MS, Roni MA, Jalil RU. Systematic development of self-emulsifying drug delivery systems of atorvastatin with improved bioavailability potential. Sci Pharm. 2012;80:1027–43.
Biswal PK, Pani NR, Dixit PK. Effects of carbohydrate polymers in self-microemulsified tablets on the bioavailability of atorvastatin: in vitro-in vivo study. Life Sci. 2015;135:92–100.
Yeom DW, Song YS, Kim SR, Kim SR, Lee SG, Song SH, et al. Development of a solidified self-microemulsifying drug delivery system (S-SMEDDS) for atorvastatin calcium with improved dissolution and bioavailability. Int J Pharm. 2016;506:302–31.
Kassem AM, Ibrahim HM, Samy AM. Development and optimization of atorvastatin calcium loaded self-nanoemulsifying drug delivery system (SNEDDS) for enhancing oral bioavailability: in vitro and in vivo evaluation. J Microencapsul. 2017;34:319–33.
Wicaksono Y, Wisudyaningsih B, Siswoyo TA. Enhancement of solubility and dissolution rate of atorvastatin calcium by co-crystallization. Trop J Pharm Res. 2017;16:1497–502.
Goellner EM, Utermoehlen J, Kramer R, Classen B. Structure of arabinogalactan from Larix laricina and its reactivity with antibodies directed against type-II-arabinogalactans. Carbohydr Polym. 2011;86:1739–44.
Trofimova NN, Medvedeva EN, Ivanova NV, Malkov YA, Babkin VA. Polysaccharides from larch biomass. In: Karunaratne DN, editor. The complex world of polysaccharides. Rijeka: InTech; 2012. p. 153–94.
Dushkin AV, Meteleva ES, Tolstikova TG, Tolstikov GA, Polyakov NE, Neverova NA, et al. Mechanochemical preparation and pharmacological activities of water-soluble intermolecular complexes of arabinogalactan with medicinal agents. Russ Chem Bull. 2008;57(6):1299–307.
Mikhailenko MA, Shakhtshneider TP, Eltsov IV, Kozlov AS, Kuznetsova SA, Karacharov АА, et al. Supramolecular architecture of betulindiacetate complexes with arabinogalactan from Larix sibirica. Carbohydr Polym. 2015;138:1–7.
Du LP, Dushkin AV, Chistyachenko YS, Polyakov NE, Su WK. Investigation the inclusion complexes of valsartan with polysaccharide arabinogalactan from larch Larix sibirica and (2-hydroxypropyl)-β-cyclodextrin: preparation, characterization and physicochemical properties. J Incl Phenom Macrocycl Chem. 2016;85:93–104.
Dushkin AV, Tolstikova TG, Khvostov MV, Tolstikov GA. Complexes of polysaccharides and glycyrrhizic acid with drug molecules. Mechanochemical synthesis and pharmacological activity. In: Karunaratne DN, editor. The complex world of polysaccharides. Rijeka: InTech; 2012. p. 573–602.
Chistyachenko YS, Dushkin AV, Tolstikova TG, Tolstikov GA, Lyakhov NZ, Polyakov NE, et al. Polysaccharide arabinogalactan from larch Larix sibirica as carrier for molecules of salicylic and acetylsalicylic acid: preparation, physicochemical and pharmacological study. Drug Deliv. 2015;22:400–7.
Chistyachenko YS, Meteleva ES, Pakharukova MY, Katokhin AV, Khvostov MV, Varlamova AI, et al. A physicochemical and pharmacological study of the newly synthesized complex of albendazole and the polysaccharide arabinogalactan from larch wood. Curr Drug Deliv. 2015;12:477–90.
Khvostov MV, Borisov SA, Tolstikova TG, Dushkin AV, Tsyrenova BD, Chistyachenko YS, et al. Supramolecular complex of ibuprofen with larch polysaccharide arabinogalactan: studies on bioavailability and pharmacokinetics. Eur J Drug Metab Pharmacokinet. 2017;42:431–40.
Matsuoka K, Miyajima R, Ishida Y, Karasawa S, Yoshimur T. Aggregate formation of glycyrrhizic acid. Colloid Surf A. 2016;500:112–7.
Dushkin AV, Meteleva ES, Tolstikova TG, Khvostov MV, Dolgikh MP. Complexing of pharmacons with glycyrrhizic acid as a route to the development of the preparations with enhanced efficiency. Chem Sustain Dev. 2010;18:437–44.
Polyakov NE, Leshina TV. Glycyrrhizic acid as a novel drug delivery vector: synergy of drug transport and efficacy. Open Conf Proc J. 2011;2:64–72.
Yasui S, Fujiwara K, Tawada A, Fukuda Y, Nakano M, Yokosuka O. Efficacy of intravenous glycyrrhizin in the early stage of acute onset autoimmune hepatitis. Dig Dis Sci. 2011;56:3638–47.
Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, et al. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;798:1494–500.
U.S. National Library of Medicine. National Center for Biotechnology Information, Glycyrrhizin. PubChem. Open Chemistry database. https://pubchem.ncbi.nlm.nih.gov/compound/Glycyrrhizic_acid#section=Top
Polyakov NE, Khan VK, Taraban MB, Leshina TV, Salakhutdinov NF, Tolstikov GA. Complexation of lappaconitine with glycyrrhizic acid: stability and reactivity studies. J Phys Chem B. 2005;109:24526–30.
Polyakov NE, Khan VK, Taraban MB, Leshina TV. Complex of calcium receptor blocker nifedipine with glycyrrhizic acid. J Phys Chem B. 2008;112:4435–40.
Polyakov NE, Leshina TV, Salakhutdinov NF, Kispert LD. Host–guest complexes of carotenoids with β-glycyrrhizic acid. J Phys Chem B. 2006;110:6991–8.
Vavilin VA, Salakhutdinov NF, Ragino YI, Polyakov NE, Taraban MB, Leshina TV, et al. The cholesterol lowering properties of the complex compound simvastatin with glycyrrhizic acid (simvaglyzin) in experimental models. Biochem Mosc Suppl Ser B. 2008;2:373–80.
Fomina MK, Avgustinovich DF, Tolstikova TG. Effects of buspirone complex with glycyrrhizic acid on behavior of mice with anxious-depressive state. Ross Fiziol Zh Im I M Sechenova. 2014;100:808–19.
Yang FH, Zhang Q, Liang QY, Wang SQ, Zhao BX, Wang YT, et al. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: paclitaxel-loaded glycyrrhizic acid micelles. Molecules. 2015;20:4337–56.
Wang YT, Zhao BX, Wang SQ, Liang QY, Cai Y, Yang FH, et al. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Deliv. 2016;23:1623–35.
Selyutina OY, Polyakov NE, Korneev DV, Zaitsev BN. Influence of glycyrrhizin on permeability and elasticity of cell membrane: perspectives for drugs delivery. Drug Deliv. 2016;23:858–65.
Boldyrev VV. Mechanochemical modification and synthesis of drugs. J Mater Sci. 2004;39:5117–20.
Dushkin AV. Potential of mechanochemical technology in organic synthesis and synthesis of new materials. Chem Sustain Dev. 2004;12:251–73.
Dushkin AV. Mechanochemical synthesis of organic compounds and rapidly soluble materials. In: Sopicka-Lizer M, editor. High-energy ball milling. Mechanochemical processing of nanopowders. Oxford: Woodhead Publishing Limited; 2010. p. 249–73.
Barzegar-Jalali M, Valizadeh H, Shadbad MRS, Adibkia K, Mohammadi G, Farahani A, et al. Cogrinding as an approach to enhance dissolution rate of a poorly water-soluble drug (gliclazide). Powder Technol. 2010;197:150–8.
Dushkin AV, Meteleva ES, Tolstikova TG, Khvostov MV. Mechanochemical preparation and properties of water-soluble intermolecular complexes of polysaccharides and β-cyclodextrin with pharmaceutical substances. Chem Sustain Dev. 2010;18:631–40.
Zhong L, Zhu XY, Luo XF, Su WK. Dissolution properties and physical characterization of telmisartan-chitosan solid dispersions prepared by mechanochemical activation. AAPS PharmSciTech. 2013;14:541–50.
Borba PA, Pinotti M, Andrade GR, Da CNJ, Olchanheski Junior LR, Fernandes D, et al. The effect of mechanical grinding on the formation, crystalline changes and dissolution behaviour of the inclusion complex of telmisartan and β-cyclodextrins. Carbohydr Polym. 2015;133:373–83.
Nart V, França MT, Anzilaggo D, Riekes MK, Kratz JM, de Campos CE, et al. Ball-milled solid dispersions of BCS class IV drugs: impact on the dissolution rate and intestinal permeability of acyclovir. Mater Sci Eng C. 2015;53:229–38.
Cugovčan M, Jablan J, Lovrić J, Cinčić D, Galić N, Jug M. Biopharmaceutical characterization of praziquantel cocrystals and cyclodextrin complexes prepared by grinding. J Pharm Biomed Anal. 2017;137:42–53.
Rasheed A, Kumar CKA, Sravanthi V. Cyclodextrins as drug carrier molecule: a review. Sci Pharm. 2008;76:567–98.
Kim JS, Kim MS, Park H, ** SJ, Lee S, Hwang SJ. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int J Pharm. 2008;359:211–9.
Eerdenbrugh BV, Speybroeck MV, Mols R, Houthoofd K, Martens JA, Froyen L, et al. Itraconazole/TPGS/Aerosil®200 solid dispersions. Characterization, physical stability and in vivo performance. Eur J Pharm Sci. 2009;38:270–8.
Di Meo C, Proietti N, Mannina L, Capitani D. NMR methodologies in the study of polysaccharides. In: Matricardi P, Alhaique F, Coviello T, editors. Polysaccharide hydrogels: characterization and biomedical applications. Singapore: Pan Stanford Publishing PTE Ltd; 2016. p. 209–43.
Apanasenko IE, SelyutinaOYu PNE, Suntsova LP, Meteleva ES, Dushkin AV, et al. Solubilization and stabilization of macular carotenoids by water soluble oligosaccharides and polysaccharides. Arch Biochem Biophys. 2015;572:58–65.
Kornievskaya VS, Kruppa AI, Polyakov NE, Leshina TV. Effect of glycyrrhizic acid on lappaconitine phototransformation. J Phys Chem B. 2007;111:11447–52.
Panakanti R, Narang AS. Impact of excipient interactions on drug bioavailability from solid dosage forms. Pharm Res. 2012;29:2639–59.
Valizadeh H, Zakeri-Milani P, Barzegar-Jalali M, Mohammadi G, Danesh-Bahreini MA, Adibkia K, et al. Preparation and characterization of solid dispersions of piroxicam with hydrophilic carriers. Drug Dev Ind Pharm. 2007;33:45–56.
Liu L, Zhu S. A study on the supramolecular structure of inclusion complex of β-cyclodextrin with prazosin hydrochloride. Carbohydr Polym. 2007;68:472–6.
Kong RP, Zhu XY, Meteleva ES, Chistyachenko YS, Suntsova LP, Polyakov NE, et al. Enhanced solubility and bioavailability of simvastatin by mechanochemically obtained complexes. Int J Pharm. 2017;534:108–18.
Zhang QH, Polyakov NE, Chistyachenko YS, Khvostov MV, Frolova TS, Tolstikova TG, et al. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018;25:198–209.
Polyakov NE, Kispert LD. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr Polym. 2015;128:207–19.
Selyutina OY, Apanasenko IE, Kim AV, Shelepova EA, Khalikov SS, Polyakov NE. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surf B Biointerfaces. 2016;147:459–66.
Moschini R, Gini F, Cappiello M, Balestri F, Falcone G, Boldrini E, et al. Interaction of arabinogalactan with mucins. Int J Biol Macromol. 2014;67:446–51.
Rong WT, Lu YP, Tao Q, Guo M, Lu Y, Ren Y, et al. Hydroxypropylsulfobutyl-β-cyclodextrin improves the oral bioavailability of edaravone by modulating drug efflux pump of enterocytes. J Pharm Sci. 2014;103:730–42.
Patel AR, Vavia PR. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007;9:344–52.
Schurr PE, Schultz JR, Parkinson TM. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids. 1972;7:68–74.
Acknowledgments
We would like to thank Ying Sun (Zhejiang University of Technology) for the help with the bioavailability experiment, Lubov Suntsova for the scanning electron microscopy analysis, and Julia Chystyachenko for the statistical calculation (Institute of Solid State Chemistry and Mechanochemistry, Russia).
Funding
This work was supported by grant 0301-2016-0018-0008 from the state assignment to ISSCM, SB RAS.