Abstract
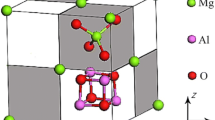
The microstructural characteristics of the CaO-SiO2-B2O3-10 mass.% MgO-30 mass.% Al2O3 systems solidified during slow cooling from 1600 °C were investigated using SEM-EDS and a thermochemical computation package. The effect of boron oxide on the crystallization behavior of the spinel in the aluminosilicate system was observed because boron oxide is believed to become a potential flux to reduce the melting point of the liquid oxides. The primary crystalline phase was spinel, mainly MgAl2O4, irrespective of the boron content. The liquidus temperature T L continuously decreased as the boron oxide content increased, indicating that the boron oxide decreased the activity of the MgAl2O4 spinel phase in liquid melts at high temperatures. The size of the spinel crystals increased as the temperature range for the solid + liquid coexisting region, viz. the mushy zone, increased. In the present systems, because the T L continuously decreased with the increase in the boron oxide content, the viscosity of the liquid oxide may have affected the crystallization behavior of the spinel during cooling. Based on these results, an injection of a small amount of B2O3 flux into molten steel containing liquid aluminosilicate inclusions is not recommended because large spinel crystals can originate from the changes in the thermophysical properties of the liquid inclusions due to the incorporation of boron oxide into the aluminosilicate networks.
Similar content being viewed by others
References
S. U. Koh, H. G. Jung, and K. B. Kang, J. Kor. Inst. Met. & Mater. 46, 257 (2008).
J. J. Kim and J. B. Lee, J. Kor. Inst. Met. & Mater. 46, 809 (2008).
H. Arai, K. Matsumoto, S. I. Shimasaki, and S. Taniguchi, ISIJ Int. 49, 965 (2009).
M. A. Van Ende, M. Guo, R. Dekkers, M. Burty, J. Van Dyck, P. T. Jones, B. Blanpain, and P. Wollant, ISIJ Int. 49, 1133 (2009).
M. Jiang, X. Wang, B. Chen, and W. Wang, ISIJ Int. 50, 95 (2010).
J. H. Park, J. Am. Ceram. Soc. 89, 608 (2006).
J. H. Park, CALPHAD 31, 428 (2007).
J. H. Park, Metall. Mater. Trans. B 38B, 657 (2007).
J. H. Park, Mater. Sci. Eng. A 472, 43 (2008).
J. H. Park and H. Todoroki, ISIJ Int. 50, 1333 (2010).
P. Rocabois, J. N. Pontoire, J. Lahmann, and H. Gaye, J. Non-Cryst. Solids 282, 98 (2001).
C. Bale, E. Belisle, P. Chartrand, S. A. Decterov, G. Eriksson, K. Hack, I. H. Jung, Y. B. Kang, J. Melancon, A. D. Pelton, C. Robelin, and S. Petersen, CALPHAD 33, 295 (2009).
J. H. Park, I. H. Jung, and S. B. Lee, Met. Mater. Int. 15, 677 (2009).
M. O. Suk and J. H. Park, J. Am. Ceram. Soc. 92, 717 (2009).
J. H. Park, J. G. Park, D. J. Min, Y. E. Lee, and Y. B. Kang, J. Eur. Ceram. Soc. 30, 3181 (2010).
J. H. Park, M. O. Suk, I. H. Jung, M. Guo, and B. Blanpain, Steel Res. Int. 81, 860 (2010).
J. H. Park, G. H. Park, and Y. E. Lee, ISIJ Int. 50, 1078 (2010).
K. Fujii, T. Nagasaka, and M. Hino, ISIJ Int. 40, 1059 (2000).
K. C. Mills, Diffusion coefficients in molten slags, in Slag Atlas, 2 nd ed., Verlag Stahleisen GmbH, Dusseldorf (1995).
R. L. A. Everman and R. F. Cooper, J. Am. Ceram. Soc. 86, 487 (2003).
J. F. Stebbins, private communication.
M. K. Naskar and M. Chatterjee, J. Eur. Ceram. Soc. 24, 3499 (2004).
S. Charkraborty, Diffusion in Silicate Melts, in Structure, Dynamics and Properties of Silicate Melts, Mineralogical Society of America, WA (1995).
D. R. Uhlmann and H. Yinnon, Glass: Science and Technology, Vol. 1, ch. 1, Academic Press, New York, NY (1983).
A. M. Sawvel, S. C. Chinn, W. L. Bourcier, and R. S. Maxwell, Chem. Mater. 17, 1493 (2005).
D. Caurant, O. Majeurus, E. Fadel, M. Lenoir, C. Gervais, and O. Pinet, J. Am. Ceram. Soc. 90, 774 (2007).
T. Sun, H. **ao, W. Guo, and X. Hong, Ceram. Int. 36, 821 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.H. The effect of boron oxide on the crystallization behavior of MgAl2O4 spinel phase during the cooling of the CaO-SiO2-10 mass.% MgO-30 mass.%Al2O3 systems. Met. Mater. Int. 16, 987–992 (2010). https://doi.org/10.1007/s12540-010-1220-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-010-1220-3