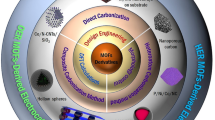
Abstract
The development of efficient and cost-effective catalysts to catalyze a wide variety of electrochemical reactions is key to realize the large-scale application of renewable and clean energy technologies. Owing to the maximum atom-utilization efficiency and unique electronic and geometric structures, single atom catalysts (SACs) have exhibited superior performance in various catalytic systems. Recently, assembled from the functionalized organic linkers and metal nodes, metal-organic frameworks (MOFs) with ultrafine porosity have received tremendous attention as precursors or self-sacrificing templates for preparing porous SACs. Here, the recent advances toward the synthesis strategies for using MOF precursors/templates to construct SACs are systematically summarized with special emphasis on the types of central metal sites. The electrochemical applications of these recently emerged MOF-derived SACs for various energy-conversion processes, such as oxygen reduction/evolution reaction (ORR/OER), hydrogen evolution reaction (HER), and CO2 reduction reaction (CO2RR), are also discussed and reviewed. Finally, the current challenges and prospects regarding the development of MOF-derived SACs are proposed.

Similar content being viewed by others
References
Wu, H. B.; Lou, X. W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252.
Wang, J. B.; Chen, W. L.; Wang, T.; Bate, N.; Wang, C. L.; Wang, E. B. A strategy for highly dispersed Mo2C/MoN hybrid nitrogen-doped graphene via ion-exchange resin synthesis for efficient electrocatalytic hydrogen reduction. Nano Res. 2018, 11, 4535–4548.
Bu, L. Z.; Shao, Q.; E, B.; Guo, J.; Yao, J. L.; Huang, X. Q. PtPb/PtNi intermetallic core/atomic layer shell octahedra for efficient oxygen reduction electrocatalysis. J. Am. Chem. Soc. 2017, 139, 9576–9582.
Chen, P. Z.; Zhou, T. P.; **ng, L. L.; Xu, K.; Tong, Y.; **e, H.; Zhang, L. D.; Yan, W. S.; Chu, W. S.; Wu, C. Z. et al. Atomically dispersed ironnitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions. Angew. Chem., Int. Ed. 2017, 56, 610–614.
Yan, X. C.; Jia, Y.; Zhang, L. Z.; Soo, M. T.; Yao, X. D. Defective graphene anchored iron-cobalt nanoparticles for efficient electrocatalytic oxygen reduction. Chem. Commun. 2017, 53, 12140–12143.
Gao, S. Y.; Geng, K. R.; Liu, H. Y.; Wei, X. J.; Zhang, M.; Wang, P.; Wang, J. J. Transforming organic-rich amaranthus waste into nitrogen-doped carbon with superior performance of the oxygen reduction reaction. Energy Environ. Sci. 2015, 8, 221–229.
Zhang, L.; Liu, B. R.; Zhang, N.; Ma, M. M. Electrosynthesis of Co3O4 and Co(OH)2 ultrathin nanosheet arrays for efficient electrocatalytic water splitting in alkaline and neutral media. Nano Res. 2018, 11, 323–333.
Sun, T. T.; Zhao, S.; Chen, W. X.; Zhai, D.; Dong, J. C.; Wang, Y.; Zhang, S. L.; Han, A. J.; Gu, L.; Yu, R. et al. Single-atomic cobalt sites embedded in hierarchically ordered porous nitrogen-doped carbon as a superior bifunctional electrocatalyst. Proc. Natl. Acad. Sci. USA 2018, 115, 12692–12697.
Yan, D. F.; Guo, L.; **e, C.; Wang, Y. Y.; Li, Y. X.; Li, H.; Wang, S. Y. N, P-dual doped carbon with trace Co and rich edge sites as highly efficient electrocatalyst for oxygen reduction reaction. Sci. China Mater. 2018, 61, 679–685.
Kuang, M.; Wang, Q. H.; Ge, H. T.; Han, P.; Gu, Z. X.; Al-Enizi, A. M.; Zheng, G. F. CuCoOx/FeOOH core–shell nanowires as an efficient bifunctional oxygen evolution and reduction catalyst. ACS Energy Lett. 2017, 2, 2498–2505.
Gupta, S.; Zhao, S.; Wang, X. X.; Hwang, S.; Karakalos, S.; Devaguptapu, S. V.; Mukherjee, S.; Su, D.; Xu, H.; Wu, G. Quaternary FeCoNiMn-based nanocarbon electrocatalysts for bifunctional oxygen reduction and evolution: Promotional role of Mn do** in stabilizing carbon. ACS Catal. 2017, 7, 8386–8393.
Dai, Z. F.; Geng, H. B.; Wang, J.; Luo, Y. B.; Li, B.; Zong, Y.; Yang, J.; Guo, Y. Y.; Zheng, Y.; Wang, X. et al. Hexagonal-phase cobalt monophosphosulfide for highly efficient overall water splitting. ACS Nano 2017, 11, 11031–11040.
Cao, J. M.; Feng, Y. Q.; Liu, B. Y.; Li, H. G. Carbon skeleton doped with Co, N, S and P as efficient electrocatalyst for oxygen evolution reaction. Sci. China Mater. 2018, 61, 686–696.
Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R. H.; Liu, S. H.; Zhuang, X. D.; Feng, X. L. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem., Int. Ed. 2016, 55, 6702–6707.
Jia, N.; Weng, Q.; Shi, Y. R.; Shi, X. Y.; Chen, X. B.; Chen, P.; An, Z. W.; Chen, Y. N-doped carbon nanocages: Bifunctional electrocatalysts for the oxygen reduction and evolution reactions. Nano Res. 2018, 11, 1905–1916.
Zhang, M. D.; Dai, Q. B.; Zheng, H. G.; Chen, M. D.; Dai, L. M. Novel MOF-derived Co@N-C bifunctional catalysts for highly efficient Zn-Air batteries and water splitting. Adv. Mater. 2018, 30, 1705431.
Wu, S. S.; Zhu, Y. G.; Hou, Y. F.; Luo, Y. C.; Zhang, L. H.; Wan, Y.; Nan, B.; Cao, L. J.; Wang, Z. Y.; Li, M. C. et al. Bimetallic organic frameworks derived CuNi/carbon nanocomposites as efficient electrocatalysts for oxygen reduction reaction. Sci. China Mater. 2017, 60, 654–663.
Qiao, Y. Y.; Yuan, P. F.; Hu, Y. F.; Zhang, J. N.; Mu, S. C.; Zhou, J. H.; Li, H.; **a, H. C.; He, J.; Xu, Q. Sulfuration of an Fe-N-C catalyst containing FexC/Fe species to enhance the catalysis of oxygen reduction in acidic media and for use in flexible Zn-Air batteries. Adv. Mater. 2018, 30, 1804504.
Song, J. F.; **ang, J. Y.; Mu, C. P.; Wang, B. C.; Wen, F. S.; Su, C.; Wang, C.; Liu, Z. Y. Facile synthesis and excellent electrochemical performance of CoP nanowire on carbon cloth as bifunctional electrode for hydrogen evolution reaction and supercapacitor. Sci. China Mater. 2017, 60, 1179–1186.
Sun, K.; Cheng, T.; Wu, L. N.; Hu, Y. F.; Zhou, J. G.; Maclennan, A.; Jiang, Z. H.; Gao, Y. Z.; Goddard, W. A., III.; Wang, Z. J. Ultrahigh mass activity for carbon dioxide reduction enabled by gold-iron core–shell nanoparticles. J. Am. Chem. Soc. 2017, 139, 15608–15611.
Yan, B.; Krishnamurthy, D.; Hendon, C. H.; Deshpande, S.; Surendranath, Y.; Viswanathan, V. Surface restructuring of nickel sulfide generates optimally coordinated active sites for oxygen reduction catalysis. Joule 2017, 1, 600–612.
Zhang, H. B.; An, P. F.; Zhou, W.; Guan, B. Y.; Zhang, P.; Dong, J. C.; Lou, X. W. Dynamic traction of lattice-confined platinum atoms into mesoporous carbon matrix for hydrogen evolution reaction. Sci. Adv. 2018, 4, eaao6657.
Stoerzinger, K. A.; Rao, R. R.; Wang, X. R.; Hong, W. T.; Rouleau, C. M.; Shao-Horn, Y. The role of Ru redox in pH-dependent oxygen evolution on rutile ruthenium dioxide surfaces. Chem 2017, 2, 668–675.
Lu, C. B.; Tranca, D.; Zhang, J.; Hernández, F. R.; Su, Y. Z.; Zhuang, X. D.; Zhang, F.; Seifert, G.; Feng, X. L. Molybdenum carbide-embedded nitrogen-doped porous carbon nanosheets as electrocatalysts for water splitting in alkaline media. ACS Nano 2017, 11, 3933–3942.
Wu, Y. S.; Liu, X. J.; Han, D. D.; Song, X. Y.; Shi, L.; Song, Y.; Niu, S. W.; **e, Y. F.; Cai, J. Y.; Wu, S. Y. et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat. Commun. 2018, 9, 1425.
Sun, T. T.; Xu, L. B.; Li, S. Y.; Chai, W. X.; Huang, Y.; Yan, Y. S.; Chen, J. F. Cobalt-nitrogen-doped ordered macro-/mesoporous carbon for highly efficient oxygen reduction reaction. Appl. Catal. B: Environ. 2016, 193, 1–8.
Su, J. W.; Ge, R. X.; Dong, Y.; Hao, F.; Chen, L. Recent progress in singleatom electrocatalysts: Concept, synthesis, and applications in clean energy conversion. J. Mater. Chem. A 2018, 6, 14025–14042.
Liu, J.; Jiao, M. G.; Lu, L. L.; Barkholtz, H. M.; Li, Y. P.; Wang, Y.; Jiang, L. H.; Wu, Z. J.; Liu, D. J.; Zhuang, L. et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938.
Sun, T. T.; Xu, L. B.; Yan, Y. S. Zakhidov, A. A.; Baughman, R. H.; Chen, J. F. Ordered mesoporous nickel sphere arrays for highly efficient electrocatalytic water oxidation. ACS Catal. 2016, 6, 1446–1450.
Tang, T.; Jiang, W. J.; Niu, S.; Liu, N.; Luo, H.; Chen, Y. Y.; **, S. F.; Gao, F.; Wan, L. J.; Hu, J. S. Electronic and morphological dual modulation of cobalt carbonate hydroxides by Mn do** toward highly efficient and stable bifunctional electrocatalysts for overall water splitting. J. Am. Chem. Soc. 2017, 139, 8320–8328.
Panda, C.; Menezes, P. W.; Walter, C.; Yao, S. L.; Miehlich, M. E.; Gutkin, V.; Meyer, K.; Driess, M. From a molecular 2Fe-2Se precursor to a highly efficient iron diselenide electrocatalyst for overall water splitting. Angew. Chem., Int. Ed. 2017, 56, 10506–10510.
Long, B.; Tang, Y.; Li, J. New mechanistic pathways for CO oxidation catalyzed by single-atom catalysts: Supported and doped Au1/ThO2. Nano Res. 2016, 9, 3868–3880.
Chen, Y. J.; Ji, S. F.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264.
Wang, X.; Chen, W. X.; Zhang, L.; Yao, T.; Liu, W.; Lin, Y.; Ju, H. X.; Dong, J. C.; Zheng, L. R.; Yan, W. S. et al. Uncoordinated amine groups of metal-organic frameworks to anchor single Ru sites as chemoselective catalysts toward the hydrogenation of quinoline. J. Am. Chem. Soc. 2017, 139, 9419–9422.
Zhang, J.; Wu, X.; Cheong, W. C.; Chen, W. X.; Lin, R.; Li, J.; Zheng, L. R.; Yan, W. S.; Gu, L.; Chen, C. et al. Cation vacancy stabilization of single-atomic-site Pt1/Ni(OH)x catalyst for diboration of alkynes and alkenes. Nat. Commun. 2018, 9, 1002.
Gao, C.; Chen, S. M.; Wang, Y.; Wang, J. W.; Zheng, X. S.; Zhu, J. F.; Song, L.; Zhang, W. K.; **ong, Y. J. Heterogeneous single-atom catalyst for visible-light-driven high-turnover CO2 reduction: The role of electron transfer. Adv. Mater. 2018, 30, 1704624.
Lin, R. H.; Albani, D.; Fako, E.; Kaiser, S. K.; Safonova, O. V.; López, N.; Pérez-Ramírez, J. Design of single gold atoms on nitrogen-doped carbon for molecular recognition in alkyne semi-hydrogenation. Angew. Chem., Int. Ed. 2019, 58, 504–509.
Qiao, B. T.; Wang, A. Q.; Yang, X. F.; Allard, L. F.; Jiang, Z.; Cui, Y. T.; Liu, J. Y.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641.
Huang, X. H.; **a, Y. J.; Cao, Y. J.; Zheng, X. S.; Pan, H. B.; Zhu, J. F.; Ma, C.; Wang, H. W.; Li, J. J.; You, R. et al. Enhancing both selectivity and coking-resistance of a single-atom Pd1/C3N4 catalyst for acetylene hydrogenation. Nano Res. 2017, 10, 1302–1312.
Zhang, C. H.; Sha, J. W.; Fei, H. L.; Liu, M. J.; Yazdi, S.; Zhang, J. B.; Zhong, Q. F.; Zou, X. L.; Zhao, N. Q.; Yu, H. S. et al. Single-atomic ruthenium catalytic sites on nitrogen-doped graphene for oxygen reduction reaction in acidic medium. ACS Nano 2017, 11, 6930–6941.
Tao, H. C.; Choi, C.; Ding, L.-X.; Jiang, Z.; Han, Z. S.; Jia, M. W.; Fan, Q.; Gao, Y. N.; Wang, H. H.; Robertson, A. W. et al. Nitrogen fixation by Ru single-atom electrocatalytic reduction. Chem 2019, 5, 204–214.
Yang, J.; Qiu, Z. Y.; Zhao, C. M.; Wei, W. C.; Chen, W. X.; Li, Z. J.; Qu, Y. T.; Dong, J. C.; Luo, J.; Li, Z. Y. et al. In situ thermal atomization to convert supported nickel nanoparticles into surface-bound nickel singleatom catalysts. Angew. Chem., Int. Ed. 2018, 57, 14095–14100.
Ahn, S. H.; Klein, M. J.; Manthiram, A. 1D Co-and N-doped hierarchically porous carbon nanotubes derived from bimetallic metal organic framework for efficient oxygen and tri-iodide reduction reactions. Adv. Energy Mater. 2017, 7, 1601979.
Wei, S. J.; Li, A.; Liu, J. C.; Li, Z.; Chen, W. X.; Gong, Y.; Zhang, Q. H.; Cheong, W. C.; Wang, Y.; Zheng, L. R. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 2018, 13, 856–861.
Fang, X. Z.; Shang, Q. C.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y. F.; Zhang, Q.; Luo, Y.; Jiang, H. L. Single Pt atoms confined into a metal-organic framework for efficient photocatalysis. Adv. Mater. 2018, 30, 1705112.
Pan, Y.; Lin, R.; Chen, Y. J.; Liu, S. J.; Zhu, W.; Cao, X.; Chen, W. X.; Wu, K. L.; Cheong, W. C.; Wang, Y. et al. Design of single-atom Co-N5 catalytic site: A robust electrocatalyst for CO2 reduction with nearly 100% CO selectivity and remarkable stability. J. Am. Chem. Soc. 2018, 140, 4218–4221.
Han, A. J.; Chen, W. X.; Zhang, S. L.; Zhang, M. L.; Han, Y. H.; Zhang, J.; Ji, S. F.; Zheng, L. R.; Wang, Y.; Gu, L. et al. A polymer encapsulation strategy to synthesize porous nitrogen-doped carbon-nanosphere-supported metal isolated-single-atomic-site catalysts. Adv. Mater. 2018, 30, 1706508.
Liang, Z. Z.; Fan, X.; Lei, H. T.; Qi, J.; Li, Y. Y.; Gao, J. P.; Huo, M. L.; Yuan, H. T.; Zhang, W.; Lin, H. P. et al. Cobalt-nitrogen-doped helical carbonaceous nanotubes as a class of efficient electrocatalysts for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2018, 57, 13187–13191.
Liang, H. W.; Brüller, S.; Dong, R. H.; Zhang, J.; Feng, X. L.; Müllen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992.
Lu, Z. Y.; Wang, B.; Hu, Y. F.; Liu, W.; Zhao, Y. F.; Yang, R. O.; Li, Z. P.; Luo, J.; Chi, B.; Jiang, Z. et al. An isolated zinc-cobalt atomic pair for highly active and durable oxygen reduction. Angew. Chem., Int. Ed. 2019, 58, 2622–2626.
Li, X. G.; Bi, W. T.; Chen, M. L.; Sun, Y. X.; Ju, H. X.; Yan, W. S.; Zhu, J. F.; Wu, X. J.; Chu, W. S.; Wu, C. Z. et al. Exclusive Ni-N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction. J. Am. Chem. Soc. 2017, 139, 14889–14892.
Zhang, Z. P.; Sun, J. T.; Wang, F.; Dai, L. M. Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework. Angew. Chem., Int. Ed. 2018, 57, 9038–9043.
Zhang, J. F.; Liu, J. Y.; **, L. F.; Yu, Y. F.; Chen, N.; Sun, S. H.; Wang, W. C.; Lange, K. M.; Zhang, B. Single-atom Au/NiFe layered double hydroxide electrocatalyst: Probing the origin of activity for oxygen evolution reaction. J. Am. Chem. Soc. 2018, 140, 3876–3879.
Chen, W. X.; Pei, J. J.; He, C. T.; Wan, J. W.; Ren, H. L.; Zhu, Y. Q.; Wang, Y.; Dong, J. C.; Tian, S. B.; Cheong, W. C. et al. Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew. Chem., Int. Ed. 2017, 56, 16086–16090.
Fei, H. L.; Dong, J. C.; Wan, C. Z.; Zhao, Z. P.; Xu, X.; Lin, Z. Y.; Wang, Y. L.; Liu, H. T.; Zang, K. T.; Luo, J. et al. Microwave-assisted rapid synthesis of graphene-supported single atomic metals. Adv. Mater. 2018, 30, 1802146.
Han, Y. H.; Wang, Y. G.; Chen, W. X.; Xu, R. R.; Zheng, L. R.; Zhang, J.; Luo, J.; Shen, R. A.; Zhu, Y. Q.; Cheong, W. C. et al. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: Superior electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2017, 139, 17269–17272.
Han, Y. H.; Wang, Y. G.; Xu, R. R.; Chen, W. X.; Zheng, L. R.; Han, A. J.; Zhu, Y. Q.; Zhang, J.; Zhang, H. B.; Luo, J. et al. Electronic structure engineering to boost oxygen reduction activity by controlling the coordination of the central metal. Energy Environ. Sci. 2018, 11, 2348–2352.
Sun, T. T.; Zhang, S. L.; Xu, L. B.; Wang, D. S.; Li, Y. D. An efficient multifunctional hybrid electrocatalyst: Ni2P nanoparticles on MOF-derived Co, N-doped porous carbon polyhedrons for oxygen reduction and water splitting. Chem. Commun. 2018, 54, 12101–12104.
Qiu, H. J.; Ito, Y.; Cong, W. T.; Tan, Y. W.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. W. Nanoporous graphene with single-atom nickel dopants: An efficient and stable catalyst for electrochemical hydrogen production. Angew. Chem., Int. Ed. 2015, 54, 14031–14035.
Sun, T. T.; Shan, N. N.; Xu, L. B.; Wang, J. X.; Chen, J. F.; Zakhidov, A. A.; Baughman, R. H. General synthesis of 3D ordered macro-/mesoporous materials by templating mesoporous silica confined in opals. Chem. Mater. 2018, 30, 1617–1624.
Ahn, S. H.; Yu, X. W.; Manthiram, A. “Wiring” Fe-Nx-embedded porous carbon framework onto 1D nanotubes for efficient oxygen reduction reaction in alkaline and acidic media. Adv. Mater. 2017, 29, 1606534.
Indra, A.; Song, T.; Paik, U. Metal organic framework derived materials: Progress and prospects for the energy conversion and storage. Adv. Mater. 2018, 30, 1705146.
Chong, L.; Wen, J. G.; Kubal, J.; Sen, F. G.; Zou, J. X.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W. J.; Liu, D. J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281.
Li, F.; Han, G. F.; Noh, H.-J.; Kim, S.-J.; Lu, Y. L.; Jeong, H. Y.; Fu, Z. P.; Baek, J.-B. Boosting oxygen reduction catalysis with abundant copper single atom active sites. Energy Environ. Sci. 2018, 11, 2263–2269.
Ye, Y. F.; Cai, F.; Li, H. B.; Wu, H. H.; Wang, G. X.; Li, Y. S.; Miao, S.; **e, S. H.; Si, R.; Wang, J. et al. Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe-N active sites for efficient oxygen and carbon dioxide electroreduction. Nano Energy 2017, 38, 281–289.
Yang, L.; Zeng, X. F.; Wang, W. C.; Cao, D. P. Recent progress in MOFderived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells. Adv. Funct. Mater. 2018, 28, 1704537.
Liu, J. L.; Zhu, D. D.; Guo, C. X.; Vasileff, A.; Qiao, S.-Z. Design strategies toward advanced MOF-derived electrocatalysts for energy-conversion reactions. Adv. Energy Mater. 2017, 7, 1700518.
Peng, Y.; Lu, B. Z.; Chen, S. W. Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Adv. Mater. 2018, 30, 1801995.
Liang, Z. B.; Qu, C.; **a, D. G.; Zou, R. Q.; Xu, Q. Atomically dispersed metal sites in MOF-based materials for electrocatalytic and photocatalytic energy conversion. Angew. Chem., Int. Ed. 2018, 57, 9604–9633.
Krivanek, O. L.; Chisholm, M. F.; Nicolosi, V.; Pennycook, T. J.; Corbin, G. J.; Dellby, N.; Murfitt, M. F.; Own, C. S.; Szilagyi, Z. S.; Oxley, M. P. et al. Atom-by-atom structural and chemical analysis by annular dark-field electron microscopy. Nature 2010, 464, 571–574.
Sun, Z. H.; Liu, Q. H.; Yao, T.; Yan, W. S.; Wei, S. Q. X-ray absorption fine structure spectroscopy in nanomaterials. Sci. China Mater. 2015, 58, 313–341.
Zhu, C. Z.; Shi, Q. R.; Xu, B. Z.; Fu, S. F.; Wan, G.; Yang, C.; Yao, S. Y.; Song, J. H.; Zhou, H.; Du, D. et al. Hierarchically porous M-N-C (M = Co and Fe) single-atom electrocatalysts with robust MNx active moieties enable enhanced ORR performance. Adv. Energy Mater. 2018, 8, 1801956.
Zheng, Y.; Jiao, Y.; Zhu, Y. H.; Cai, Q. R.; Vasileff, A.; Li, L. H.; Han, Y.; Chen, Y.; Qiao, S. Z. Molecule-level g-C3N4 coordinated transition metals as a new class of electrocatalysts for oxygen electrode reactions. J. Am. Chem. Soc. 2017, 139, 3336–3339.
Fei, H. L.; Dong, J. C.; Arellano-Jiménez, M. J.; Ye, G. L.; Kim, N. D.; Samuel, E. L. G.; Peng, Z. W.; Zhu, Z.; Qin, F.; Bao, J. M. et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668.
Tao, L.; Lin, C.-Y.; Dou, S.; Feng, S.; Chen, D. W.; Liu, D. D.; Huo, J.; **a, Z. H.; Wang, S. Y. Creating coordinatively unsaturated metal sites in metal-organic-frameworks as efficient electrocatalysts for the oxygen evolution reaction: Insights into the active centers. Nano Energy 2017, 41, 417–425.
Zhao, W. P.; Wan, G.; Peng, C. L.; Sheng, H. P.; Wen, J. G.; Chen, H. R. Key single-atom electrocatalysis in metal-organic framework (MOF)-derived bifunctional catalysts. ChemSusChem 2018, 11, 3473–3479.
Wang, X. Q.; Chen, Z.; Zhao, X. Y.; Yao, T.; Chen, W. X.; You, R.; Zhao, C. M.; Wu, G.; Wang, J.; Huang, W. X. et al. Regulation of coordination number over single Co sites: Triggering the efficient electroreduction of CO2. Angew. Chem., Int. Ed. 2018, 57, 1944–1948.
Yin, P. Q.; Yao, T.; Wu, Y. E.; Zheng, L. R.; Lin, Y.; Liu, W.; Ju, H. X.; Zhu, J. F.; Hong, X.; Deng, Z. X. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805.
Wang, X. X.; Cullen, D. A.; Pan, Y. T.; Hwang, S.; Wang, M. Y.; Feng, Z. X.; Wang, J. Y.; Engelhard, M. H.; Zhang, H. G.; He, Y. H. et al. Nitrogen-coordinated single cobalt atom catalysts for oxygen reduction in proton exchange membrane fuel cells. Adv. Mater. 2018, 30, 1706758.
He, Y. H.; Hwang, S.; Cullen, D. A.; Uddin, M. A.; Langhorst, L.; Li, B. Y.; Karakalos, S.; Kropf, A. J.; Wegener, E. C.; Sokolowski, J. et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2019, 12, 250–260.
Chen, Y. J.; Ji, S. F.; Wang, Y. G.; Dong, J. C.; Chen, W. X.; Li, Z.; Shen, R. A.; Zheng, L. R.; Zhuang, Z. B.; Wang, D. S. et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2017, 56, 6937–6941.
Jiang, R.; Li, L.; Sheng, T.; Hu, G. F.; Chen, Y. G.; Wang, L. Y. Edge-site engineering of atomically dispersed Fe-N4 by selective C–N bond cleavage for enhanced oxygen reduction reaction activities. J. Am. Chem. Soc. 2018, 140, 11594–11598.
Zhang, H. G.; Hwang, S.; Wang, M. Y.; Feng, Z. X.; Karakalos, S.; Luo, L. L.; Qiao, Z.; **e, X. H.; Wang, C. M.; Su, D. et al. Single atomic iron catalysts for oxygen reduction in acidic media: Particle size control and thermal activation. J. Am. Chem. Soc. 2017, 139, 14143–14149.
Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S. H.; Jiang, H. L. From metalorganic frameworks to single-atom Fe implanted N-doped porous carbons: Efficient oxygen reduction in both alkaline and acidic media. Angew. Chem., Int. Ed. 2018, 57, 8525–8529.
Zhao, C. M.; Dai, X. Y.; Yao, T.; Chen, W. X.; Wang, X. Q.; Wang, J.; Yang, J.; Wei, S. Q.; Wu, Y. E.; Li, Y. D. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081.
Fan, L. L.; Liu, P. F.; Yan, X. C.; Gu, L.; Yang, Z. Z.; Yang, H. G.; Qiu, S. L.; Yao, X. D. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun. 2016, 7, 10667.
Qu, Y. T.; Li, Z. J.; Chen, W. X.; Lin, Y.; Yuan, T. W.; Yang, Z. K.; Zhao, C. M.; Wang, J.; Zhao, C.; Wang, X. et al. Direct transformation of bulk copper into copper single sites via emitting and trap** of atoms. Nat. Catal. 2018, 1, 781–786.
Li, J. Z.; Chen, M. J.; Cullen, D. A.; Hwang, S.; Wang, M. Y.; Li, B. Y.; Liu, K. X.; Karakalos, S.; Lucero, M.; Zhang, H. G. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945.
Chen, W. X.; Pei, J. J.; He, C. T.; Wan, J. W.; Ren, H. L.; Wang, Y.; Dong, J. C.; Wu, K. L.; Cheong, W. C.; Mao, J. J. et al. Single tungsten atoms supported on MOF-derived N-doped carbon for robust electrochemical hydrogen evolution. Adv. Mater. 2018, 30, 1800396.
Wang, J.; Huang, Z. Q.; Liu, W.; Chang, C. R.; Tang, H. L.; Li, Z. J.; Chen, W. X.; Jia, C. J.; Yao, T.; Wei, S. Q. et al. Design of N-coordinated dualmetal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284.
Zhang, D. Y.; Chen, W. X.; Li, Z.; Chen, Y. J.; Zheng, L. R.; Gong, Y.; Li, Q. H.; Shen, R. A.; Han, Y. H.; Cheong, W. C. et al. Isolated Fe and Co dual active sites on nitrogen-doped carbon for a highly efficient oxygen reduction reaction. Chem. Commun. 2018, 54, 4274–4277.
Zhang, L. Z.; Fischer, J. M. T. A.; Jia, Y.; Yan, X. C.; Xu, W.; Wang, X. Y.; Chen, J.; Yang, D. J.; Liu, H. W.; Zhuang, L. Z. et al. Coordination of atomic Co-Pt coupling species at carbon defects as active sites for oxygen reduction reaction. J. Am. Chem. Soc. 2018, 140, 10757–10763.
Lai, Q. X.; Zheng, L. R.; Liang, Y. Y.; He, J. P.; Zhao, J. X.; Chen, J. H. Metal–organic-framework-derived Fe-N/C electrocatalyst with five-coordinated Fe-Nx sites for advanced oxygen reduction in acid media. ACS Catal. 2017, 7, 1655–1663.
Chen, Y. J.; Ji, S. F.; Zhao, S.; Chen, W. X.; Dong, J. C.; Cheong, W. C.; Shen, R. A.; Wen, X. D.; Zheng, L. R.; Rykov, A. I. et al. Enhanced oxygen reduction with single-atomic-site iron catalysts for a zinc-air battery and hydrogen-air fuel cell. Nat. Commun. 2018, 9, 5422.
Zhu, Q.-L.; **a, W.; Zheng, L.-R.; Zou, R. Q.; Liu, Z.; Xu, Q. Atomically dispersed Fe/N-doped hierarchical carbon architectures derived from a metal–organic framework composite for extremely efficient electrocatalysis. ACS Energy Lett. 2017, 2, 504–511.
Wei, L.; Karahan, H. E.; Zhai, S. L.; Liu, H. W.; Chen, X. C.; Zhou, Z.; Lei, Y. J.; Liu, Z. W.; Chen, Y. Amorphous bimetallic oxide-graphene hybrids as bifunctional oxygen electrocatalysts for rechargeable Zn-Air batteries. Adv. Mater. 2017, 29, 1701410.
Chao, T. T.; Luo, X.; Chen, W. X.; Jiang, B.; Ge, J. J.; Lin, Y.; Wu, G.; Wang, X. Q.; Hu, Y. M.; Zhuang, Z. B. et al. Atomically dispersed copperplatinum dual sites alloyed with palladium nanorings catalyze the hydrogen evolution reaction. Angew. Chem., Int. Ed. 2017, 56, 16047–16051.
Jiang, K. Z.; Wang, P. T.; Guo, S. J.; Zhang, X.; Shen, X.; Lu, G.; Su, D.; Huang, X. Q. Ordered PdCu-based nanoparticles as bifunctional oxygenreduction and ethanol-oxidation electrocatalysts. Angew. Chem., Int. Ed. 2016, 55, 9030–9035.
Zhao, R.; Liang, Z. B.; Gao, S.; Yang, C.; Zhu, B. J.; Zhao, J. L.; Qu, C.; Zou, R. Q.; Xu, Q. Puffing up energetic metal-organic frameworks to large carbon networks with hierarchical porosity and atomically dispersed metal sites. Angew. Chem., Int. Ed. 2019, 58, 1975–1979.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2016YFA0202801), the National Natural Science Foundation of China (Nos. 21671117, 21871159, 21890383, and 21676018), and the China Postdoctoral Science Foundation (No. 2017M610864).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, T., Xu, L., Wang, D. et al. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res. 12, 2067–2080 (2019). https://doi.org/10.1007/s12274-019-2345-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2345-4