Abstract
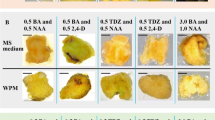
This study investigated the induction and in vitro alkaloid yield of calluses and protocorm-like bodies (PLBs) from Pinellia ternata (Thunb.) Berit (Araceae). We planned to use this material in future studies related to the mass production of medicinally valuable compounds and regulation of alkaloid metabolism. Different combinations of 2,4-dichlorophenoxyacetic acid (2,4-D), 6-benzyladenine (6-BA), kinetin (Kin), and α-naphthaleneacetic acid (NAA) were used to induce callus and PLB formation from P. ternata tuber explants. The results showed that three physiologically distinct calluses were induced by different combinations of 2,4-D, 6-BA, and Kin used in this study. The calluses differed in color, texture, differentiation status, and alkaloid content. The alkaloid content of the three calli types ranged from 0.0175% to 0.0293%. In comparison, the alkaloid content of field-grown tubers was 0.0072%. Many reports have indicated that 2,4-D suppresses the biosynthesis of secondary metabolites; however, our results show that 2,4-D promoted alkaloid production in Pinellia calluses. The combination of NAA + 6-BA induced PLB formation. The PLB alkaloid content of 0.0321% was 1.1 to 1.8 times higher than the alkaloid content of the calluses and 4.5 times higher than the field-grown tubers. In conclusion, the induction of calluses and PLBs with alkaloid content greater than that of field-grown tubers indicates the potential use of these tissue culture materials for bioprocessing alkaloids from P. ternata and for the study of alkaloid metabolism.


Similar content being viewed by others
References
Dias A. C. P.; Seabra R. M.; Andrade P. B. Xanthone biosynthesis and accumulation in calli and suspended cells of Hypericum androsaemum. Plant Sci 150: 93–101; 2000.
Dörnenburg H.; Seydel P. Effect of irradiation intensity on cell growth and kalata B1 accumulation in Oldenlandia affinis cultures. Plant Cell Tissue Organ Cult 92: 93–99; 2008.
Gibson D. M.; Ketchum R. E. B.; Vance N. C.; Christen A. A. Initiation and growth of cells lines of Taxus brevifolia (Pacific yew). Plant Cell Rep 12: 479–482; 1993.
Han M. H.; Yang X. W.; Zhang M.; Zhong G. Y. Phytochemical study of the rhizome of Pinellia ternata and quantification of phenylpropanoids in commercial Pinellia tuber by RP-LC. Chromatographia 64: 647–653; 2006.
He Y. K.; Liu G.; Lu T. G.; Sun J. S. Stem apex culture and quality improvement of tubercles of Pinellia ternata. Acta Bot Sin 36: 39–44; 1994.
He Y. K.; Zhu C. F.; He M. Y.; Hao S. Protoplast culture and plant regeneration of Pinellia ternata. Plant Cell Rep 16: 92–96; 1996.
Holden M. A. Elicitation of cell cultures. In: Robins R. J.; Rhodes M. J. C. (eds) Manipulating Secondary Metabolism in Culture. Cambridge University Press, Cambridge, pp 57–65; 1988.
Hu X. F.; Ying F. X.; He Y. B.; Gao Y. Y. Characterization of Pectobacterium carotovorum subsp. carotovorum causing soft-rot disease on Pinellia ternata in China. Eur J Plant Pathol 120: 305–310; 2008.
Kutchan T. Alkaloid biosynthesis—the basis for metabolic engineering of medicinal plants. Plant Cell 7: 1059–1070; 1995.
Lucumi E.; Luczkiewicz M.; Vera A.; Hallard D.; Heijden R. V. D.; Verpoorte R. Alkaloid formation in cell suspension cultures of Tabernaemontana divaricata after feeding of tryptamine and loganin. Biotechnol Lett 23: 1691–1696; 2001.
Magdi E. S.; Young H. C.; Frederich M.; Sittiruk R.; Robert V. Alkaloid accumulation in Catharanthus roseus cell suspension cultures fed with stemmadenine. Biotechnol Lett 26: 793–798; 2004.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–479; 1962.
Niamh A. O. D.; Patrick G. M. C.; David H. S. R.; Graham W. Callus production, suspension culture and in vitro alkaloid yields of Ephedra. Plant Cell Tissue Organ Cult 34: 149–155; 1993.
Rhodes M. J. C.; Parr A. J.; Giulietti A. Influence of exogenous hormones on the growth and secondary metabolite formation in transformed root culture. Plant Cell Tissue Organ Cult 38: 143–151; 1994.
Rokem J. S.; Goldberg I. Secondary metabolites from plant cell suspension cultures: methods for yield improvement. Adv Biotechnol Process 4: 241–274; 1985.
Shoyama Y.; Nishioka I.; Hatano K. In: Bajaj Y. P. S. (ed) Biotechnology in Agriculture and Forestry 19, High-tech and micropropagation Ш. Springer-Verlag, Berlin, Germany, pp 464–480; 1992.
Suk W. K.; Dong S. I.; Kyoung H. T.; Jang R. L. Somatic embryogenesis and plant regeneration in leaf and petiole explant cultures and cell suspension cultures of Pinellia tripartite. Plant Cell Tissue Organ Cult 80: 267–270; 2005.
Tsay H. S.; Gau T. G.; Chen C. C. Rapid clonal propagation of Pinellia ternata by tissue culture. Plant Cell Rep 8: 450–454; 1989.
Xu J. F.; Zhao Y.; Han A.; Feng P. Induction and culture of calli from Rhodiola sachalinesis A. Bor. Chin J Appl Environ Biol 1: 19–25; 1995.
Xu T. F.; Zhang L.; Sun X. F.; Tang K. X. Efficient in vitro plant regeneration of Pinellia ternata (Thunb) Breit. Acta Biol Crac Ser Bot 2: 27–32; 2005.
Yu C.; Zhang M.; Wang Y.; Luo Q. Determination of alkaloids content of cultivated and wild Pinellia ternata from various areas. Chin Mater Med 29: 583–584; 2004.
Zeng J. H.; Peng Z. S.; Mao Z. C.; Wei S. H. Study on optimum extration conditions of alkaloids from Pinellia ternata. Chin Mater Med 26: 361–363; 2003.
Zhang K. W.; Wu H.; Li W. Determination of inosine and guanosine in Rhizoma Pinellia ternata. Chin J Pharm Anal 25: 487–489; 2005.
Zhao J.; Zhu W. H.; Hu Q. Effects of light and plant growth regulators on the biosynthesis of vindoline and other indole alkaloids in Catharanthus roseus callus cultures. Plant Growth Regul 33: 43–49; 2001.
Acknowledgments
This work was supported by a grant to Prof. Liang from the Knowledge Innovation Project of Chinese Academy of Sciences (no. K2 CX2-XB1-05). We gratefully acknowledge Associate Professor Xu Hong and the technical staff in the Department of Bioscience for their assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: F. A. Krens
Rights and permissions
About this article
Cite this article
Liu, Y., Liang, Z. & Zhang, Y. Induction and in vitro alkaloid yield of calluses and protocorm-like bodies (PLBs) from Pinellia ternata . In Vitro Cell.Dev.Biol.-Plant 46, 239–245 (2010). https://doi.org/10.1007/s11627-009-9268-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-009-9268-9