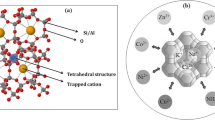
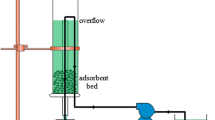
Abstract
Many industries discharge wastewater from processing into surface and underground waterways, and then, these waste waters must therefore be treated in order to remove heavy metals. The most common treatment used is the activated carbon adsorption, a particularly competitive and effective process; however, the use of activated carbon is not suitable due to the high costs. Then, in order to minimize processing cost, recent investigations have been focused on the use of low-cost adsorbents as zeolites. In particular, clinoptilolite is known to have high selectivity for certain heavy metals. In this paper, the capability of clinoptilolite as a low-cost adsorbent for the removal of zinc and cadmium ions from wastewater was analyzed in a batch system. Preliminary characterization was performed on adsorbent material in order to evaluate the chemical-physical structure. Tests in batch for analyzing adsorbing capacity of clinoptilolite were carried out varying zinc and cadmium concentrations between 10 and 200 mg/L with different amounts of sorbent in the solution (10–60 g/L). For both zinc and cadmium ions, complete adsorption was reached when the concentration was equal to 10 mg/L and adsorption capacity decreased increasing metals amount. In particular, clinoptilolite permitted high Cd2+ abatement, probably due to its greater affinity with adsorbent in the single system. Binary system was then analyzed, and, contrary to previous tests, the adsorbent in the simultaneous presence of the two metals demonstrated a greater affinity toward zinc, showing a higher percentage of absorption, due to a different absorption mechanism in the presence of two ions.







Similar content being viewed by others
References
Barros MASD et al (2003) Binary ion exchange of metal ions in Y and X zeolites. Braz J Chem Eng 20:413–421
Bisson M, Houeix I (2014) INERIS - Fiche de données toxicologiques et environnementales des substances chimiques - Cadmium et ses dérivés. DRC-11-117259-10308B.doc
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18:1501–1507
Curkovic L et al (1997) Metal ion exchange by natural and modified zeolites. Water Res 31:1379–1382
Directive 2000/11/ CE of the European Parliament. https://eur-lex.europa.eu/
Directive 2000/60/CE of the European Parliament. https://eur-lex.europa.eu/
EPA’s Chemical Ranking Report for the RCRA PBT List Docket (1998) Persistent, Bioaccumulation and toxic chemicals. https://files.nc.gov/ncdeq/document-library/PBTfact.pdf
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
European Commission (2001) Pollutants in urban wastewater and sewage sludge final report. https://ec.europa.eu/environment/archives/waste/sludge/pdf/sludge_pollutants_xsum.pdf
Gorimbo J, Taenzana B, Muleja AA (2018) Adsorption of cadmium, nickel and lead ions: equilibrium, kinetic and selectivity studies on modified clinoptilolites from the USA and RSA. Environ Sci Pollut Res 25:30962–30978
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Kesraoui-Ouki et al (1994) Natural zeolite utilisation in pollution control: a review of applications to metals’ effluents. J Chem Technol Biotechnol 59:121–126
Leppert D (1990) Heavy metal sorption with clinoptilolite zeolite. Alternatives for treating contaminated soil and water. Min Eng 42:604–608
Lewis MD (1981) Clinoptilolite, as a N, K, and Zn source for plants. M.S. thesis, Colorado State University, Fort, Collins
Liu W, Singh RP, Jothivel S (2019) Evaluation of groundwater hardness removal using activated clinoptilolite. Environ Sci Pollut Res 1–9
MINAS (2001) Pollution control acts, rules, notification issued thereunder Central Pollution Control Board, Ministry of Environment and Forests, Govt. of India, New Delhi
Mohan D, Singh KP (2002) Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse–an agricultural waste. Water Res 36:2304–2318
Mohana D, Pittman CJ (2006) Activated carbons and low-cost adsorbents for remediation of tri- and hexavalent chromium from water. J Hazard Mater B 137:762–811
Panday KK, Parsed G, Singh VN (1985) Copper (II) removal from aqueous solutions by fly ash. Water Res 19:869–873
Perrin TS, Drost DT, Boettinger JL, Norton JM (1998) Ammonium-loaded clinoptilolite: a slow-release nitrogen fertilizer for sweet corn. J Plant Nutr 21:515–530
Piumetti M, Russo N (2017) Notes on catalysis for environment and energy. CLUT, Torino
Sellaoui L, Dotto GL, Lamine AB, Erto A (2017) Interpretation of single and competitive adsorption of cadmium and zinc on activated carbon using monolayer and exclusive extended monolayer models. Environ Sci Pollut Res Int 24:19902–19908
Shaheen SM et al (2012) Removal of heavy metals from aqueous solution by zeolite in competitive sorption system. Int J Environ Sci Technol 3:362–367
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Sprynskyy M, Buszewski B, Terzyk AP, Namieśnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J Colloid Interface Sci 304:21–28
Srivastava VC et al (2008) Removal of cadmium (II) and zinc (II) metal ions from binary aqueous solution by rice husk ash. Colloid Sur A 312:172–184
Water Quality for Ecosystem and Human Health (2008) United Nations Environment Programme Global Environment Monitoring System/Water Programme. Burlington, Ontario
WHO Guidelines for Drinking-water Quality (2011) World Health Organization
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editer: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galletti, C., Dosa, M., Russo, N. et al. Zn2+ and Cd2+ removal from wastewater using clinoptilolite as adsorbent. Environ Sci Pollut Res 28, 24355–24361 (2021). https://doi.org/10.1007/s11356-020-08483-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08483-z