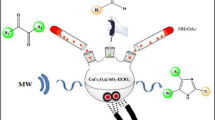
Abstract
A magnetically separable nanocatalyst prepared by incorporating Pd nanoparticles onto CoFe2O4 magnetic nanoparticles was found to be very effective in catalyzing Sonogashira cross-coupling reactions. In this green synthetic process, it is not necessary to use an external linker to support the palladium nanoparticles onto the cobalt ferrite matrix. The catalyst is effective without the use of any ligand or copper additive. The reaction works smoothly in ethanol at 70 °C with both aryl iodides and bromides to produce corresponding product in high yield. After completion of the reaction, the catalyst could be easily separated using an external magnet and reused up to five catalytic cycles with sustained catalytic activity.







Similar content being viewed by others
References
M.B. Gawande, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42, 3371–3393 (2013)
Y. Zhu, L.P. Stubbs, F. Ho, R. Liu, C.P. Ship, J.A. Maguire, N.S. Hosmane, ChemCatChem 2, 365–374 (2010)
A.-H. Lu, E.L. Salabas, F. Schuth, Angew. Chem. Int. Ed. Engl. 46, 1222–1244 (2007)
K.V.S. Ranganath, J. Kloesges, A.H. Schafer, F. Glorius, Angew. Chem. Int. Ed. Engl. 49, 7786–7789 (2010)
N.T.S. Phan, H.V. Le, J. Mol. Catal. A 334, 130–138 (2011)
K.K. Dey, K.K. Senapati, P. Phukan, S. Basu, A. Chattopadhyay, J. Phys. Chem. C 115, 12708–12715 (2011)
K.K. Senapati, S. Roy, P. Phukan, J. Mol. Catal. A 352, 128–134 (2012)
A. Serdar, K. Murat, V. Muervet, O. Saim, Appl. Catal. B 147, 387–393 (2014)
M. Beygzadeh, A. Alizadeh, M.M. Khodaei, D. Kordestani, Catal. Commun. 32, 86–91 (2013)
B.R. Vaddula, A. Saha, J. Leazer, R.S. Varma, Green Chem. 14, 2133–2136 (2012)
K. Park, J.-M. You, S. Jeon, S. Lee, Eur. J. Org. Chem. 1973–1978 (2013)
M.S. Maji, S. Murarka, A. Studer, Org. Lett. 12, 3878–3881 (2010)
K. Sonogashira, J. Organomet. Chem. 653, 46–49 (2002)
T. Mino, S. Suzuki, K. Hirai, M. Sakamoto, T. Fujita, Synlett, 3054–3131 (2011)
R. Chinchilla, C. Najera, Chem. Soc. Rev. 40, 5084–5121 (2011)
K. Sonogashira, Y. Tohda, N. Hagihara, Tetrahedron Lett. 16, 4467–4470 (1975)
G. Evano, N. Blanchard, M. Toumi, Chem. Rev. 108, 3054–3131 (2008)
J.-H. Li, Y. Liang, Y.-X. **e, J. Org. Chem. 70, 4393–4396 (2005)
E. Buxaderas, D.A. Alonso, C. Nájera, Eur. J. Org. Chem. 5864–5870 (2013)
A. Soheili, J. Murry, J.A. Albaneze-Walker, P.G. Dormer, D.L. Hughes, Org. Lett. 5, 4191–4194 (2003)
M. Larhed, A. Hallberg, J. Org. Chem. 61, 9582–9584 (1996)
A. Arques, D. Aunon, P. Molina, Tetrahedron Lett. 45, 4337–4340 (2004)
J. Cheng, Y. Sun, F. Wang, M. Guo, J.-H. Xu, Y. Pan, Z. Zhang, J. Org. Chem. 69, 5428–5432 (2004)
B.H. Lipshutz, D.W. Chung, B. Rich, Org. Lett. 10, 3793–3796 (2008)
L.-X. Shao, M. Shi, J. Org. Chem. 70, 8635–8637 (2005)
A.O. King, N. Yasuda, Top. Organomet. Chem. 6, 205–245 (2004)
R. Chinchilla, C. Najera, Chem. Rev. 107, 874–922 (2007)
J. Hassan, M. Sevignon, Ci. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 102, 1359–1470 (2002)
E. Negishi, Handbook of Organopalladium Chemistry for Organic Synthesis (Wiley-Interscience, New York, 2002), pp. 229–409
N. Miyaura, A. Suzuki, Chem. Rev. 95, 2457–2483 (1995)
N.T.S. Phan, M.V.D. Sluys, C.W. Jones, Adv. Synth. Catal. 348, 609–679 (2006)
L. Xue, Z. Lin, Chem. Soc. Rev. 39, 1692–1705 (2010)
A.M. Trzeciak, J.J. Ziolkowski, Coord. Chem. Rev. 249, 2308–2322 (2005)
P. Das, U. Bora, A. Tairai, C. Sharma, Tetrahedron Lett. 51, 1479–1482 (2010)
D. Mujahidin, S. Doye, Eur. J. Org. Chem. 2689–2693 (2005)
A. Molnar, Chem. Rev. 111, 2251–2320 (2011)
D. Astruc, Inorg. Chem. 46, 1884–1894 (2007)
Z. Yinghuai, S.C. Peng, A. Emi, S. Zhenshum, R.K. Monalisa, Adv. Synth. Catal. 349, 1917–1922 (2007)
L. Yin, J. Liebscher, Chem. Rev. 107, 133–173 (2007)
M. Lamblin, L. Nassar-Hardy, J.-C. Hierso, E. Fouquet, F.Å.-X. Felpin, Adv. Synth. Catal. 352, 33–79 (2010)
A. Modak, J. Mondal, A. Bhaumik, Green Chem. 14, 2840–2855 (2012)
C. Cai, Y. He, J. Organomet. Chem. 696, 2689–2692 (2011)
A.S. Roy, J. Mondal, B. Banerjee, P. Mondal, A. Bhaumik, S.M. Islam, Appl. Catal. A 469, 320–327 (2014)
P. Veerakumar, M. Velayudham, K.-L. Lu, S. Rajagopal, Appl. Catal. A 455, 247–260 (2013)
L.-M. Tan, Z.-Y. Sem, W.-Y. Chong, X. Liu, Hendra, W.L. Kwan, C.-L.K. Lee, Org. Lett. 15, 65–67 (2013)
B.J. Borah, D.K. Dutta, J. Mol. Catal. A 366, 202–209 (2013)
B.N. Lin, S.-H. Huang, W.-Y. Wu, C.-Y. Mou, F.-Y. Tsai, Molecules 15, 9157–9173 (2010)
P. Dutta, A. Sarkar, Adv. Synth. Catal. 353, 2814–2822 (2011)
S. El Hankari, A. El Kadib, A. Finiels, A. Bouhaouss, J.J.E. Moreau, C.M. Crudden, D. Brunel, P. Hesemann, Chem. Eur. J. 17, 8984–8994 (2011)
M.-J. **, D.-H. Lee, Angew. Chem. Int. Ed. Engl. 49, 1119–1122 (2010)
M. Choi, D.-H. Lee, K. Na, B.-W. Yu, R. Ryoo, Angew. Chem. Int. Ed. Engl. 48, 3673–3676 (2009)
K. Komura, H. Nakamura, Y. Sugi, J. Mol. Catal. A 293, 72–78 (2008)
C. Gonzalez-Arellano, A. Abad, A. Corma, H. Garcia, M. Iglesias, F. Sanchez, Angew. Chem. Int. Ed. Engl. 46, 1536–1538 (2007)
M.B. Thathagar, G. Rothenberg, Org. Biomol. Chem. 4, 111–115 (2006)
K.M. Dawood, W. Solodenko, A. Kirschning, ARKIVOC V, 104–124 (2007)
P. Rollet, W. Kleist, V. Dufaud, L. Djakovitch, J. Mol. Catal. A 241, 39–51 (2005)
N. Kim, M.S. Kwon, C.M. Park, J. Park, Tetrahedron Lett. 45, 7057–7059 (2004)
L. Djakovitch, P. Rollet, Tetrahedron Lett. 45, 1367–1370 (2004)
K.K. Senapati, C. Borgohain, P. Phukan, J. Mol. Catal. A 339, 24 (2011)
K.K. Senapati, C. Borgohain, K.C. Sarma, P. Phukan, J. Mol. Catal. A 346, 111–116 (2011)
C. Borgohain, K.K. Senapati, K.C. Sarma, P. Phukan, J. Mol. Catal. A 363–364, 495–500 (2012)
K.K. Senapati, C. Borgohain, P. Phukan, Catal. Sci. Technol. 2361–2366 (2012)
C. Luo, Y. Zhang, Y. Wang, J. Mol. Catal. A 229, 7–12 (2005)
C. Borgohain, K.K. Senapati, D. Mishra, K.C. Sarma, P. Phukan, Nanoscale 2, 2250–2256 (2010)
X. Jia, D. Chen, X. Jiao, T. He, H. Wang, W. Jiang, J. Phys. Chem. C 112, 911–917 (2008)
P. Vlazan, S.F. Rus, I. Grozescu, E. Vasile, Phys. Scr. T 157, 014047 (2013)
M. Kaya, M. Zahmakiran, S. Özkar, M. Volkan, ACS Appl. Mater. Interfaces 4, 3866–3873 (2012)
S. Akbayrak, M. Kaya, M. Volkan, S. Özkar, Appl. Catal. B 147, 387–393 (2004)
B.D. Cullity, Elements of X-ray diffraction (Addison-Wesley, London, 1959), p. 261
Acknowledgments
Financial support from DST, India (Grant No. SR/NM/NS-18/2011) is gratefully acknowledged. SR thanks UGC for a research fellowship. The authors would also like to acknowledge the support from SAIF-GU, SAIF-NEHU, and CIF of IIT Guwahati for analytical facilities during the course of investigations.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roy, S., Senapati, K.K. & Phukan, P. Direct use of nanoparticles as a heterogeneous catalyst: Pd0-doped CoFe2O4 magnetic nanoparticles for Sonogashira coupling reaction. Res Chem Intermed 41, 5753–5767 (2015). https://doi.org/10.1007/s11164-014-1699-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1699-1