Abstract
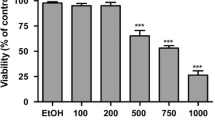
Acacetin, a bioflavanoid, contains anti-inflammatory and anti-cancer activities as shown in different experimental models. However, its anticancer potential and mechanism of action against colorectal cancer cells is largely unknown. Here, we have investigated the efficacy of acacetin using two colorectal adenocarcinoma SW480 and HCT-116 cell lines. Cell survival was examined by Trypan-blue exclusion and MTT assays, cell cycle analysis by FACS, apoptosis was assessed using Annexin V FITC assay and nuclear condensation by Hoechst staining, ROS level by DCFDA and Mitosox, and protein expression level by Western blotting. Acacetin reduced the cell survival and proliferation of both types of cells, and induced S- and G2-M phase arrest and also reduced the levels of β-catenin and its downstream target c-myc. Further, acacetin induced apoptosis as examined by Annexin-V FITC and nuclear condensation. It increased intracellular ROS production, especially mitochondrial ROS. Acacetin increased mitochondrial membrane potential depolarization and Bax:Bcl-2 ratio. Although significant changes in caspases -8 and -9 and PARP level was not observed, acacetin could induce the truncation and subsequent translocation of activated AIF from mitochondria to cytosol, which could further induce chromosomal breakage leading to apoptosis. In conclusion, Acacetin induces mitochondrial ROS-mediated cell death in a caspase-independent manner in SW480 and HCT-116 colon carcinoma cells by inducing apoptosis inducing factor (AIF), which may potentiate its anticancer and chemotherapeutic prospects against colorectal carcinoma.








Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66:683–691
Compton CC, Greene FL (2004) The staging of colorectal cancer: 2004 and beyond. CA 54(6):295–308
Czadek P (2016) Chemoprevention of colorectal cancer. Pol Ann Med 23:75–79
Pan M-H, Lai C-S, Hsu P-C, Wang Y-J (2005) Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agric Food Chem 53:620–630
Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R (2005) Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure–activity relationship with linarin and linarin acetate. Carcinogenesis 26:845–854
Hsu Y-L, Kuo P-L, Liu C-F, Lin C-C (2004) Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett 212:53–60
Shim H-Y, Park J-H, Paik H-D, Nah S-Y, Kim DS, Han YS (2007) Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol Cells 24:95–104
Zhang Q, Zhao X-H, Wang Z-J (2009) Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G 2/M arrest and apoptosis. Toxicol In Vitro 23:797–807
Fearon ER, Dang CV (1999) Cancer genetics: tumor suppressor meets oncogene. Curr Biol 9:R62–R65
Sabarwal A, Agarwal R, Singh RP (2017) Fisetin inhibits cellular proliferation and induces mitochondria-dependent apoptosis in human gastric cancer cells. Mol Carcinog 56:499–514
Tailor D, Hahm E-R, Kale RK, Singh SV, Singh RP (2014) Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion 16:55–64
Pladzyk A, Reddy AB, Yadav UC, Tammali R, Ramana KV, Srivastava SK (2006) Inhibition of aldose reductase prevents lipopolysaccharide-induced inflammatory response in human lens epithelial cells. Invest Ophthalmol Vis Sci 47:5395–5403
Agraval H, Yadav UCS (2019) MMP-2 and MMP-9 mediate cigarette smoke extract-induced epithelial-mesenchymal transition in airway epithelial cells via EGFR/Akt/GSK3β/β-catenin pathway: amelioration by fisetin. Chem Biol Interact 314:108846
Henriksson ML et al (2011) Colorectal cancer cells activate adjacent fibroblasts resulting in FGF1/FGFR3 signaling and increased invasion. Am J Pathol 178(3):1387–1394
Mukhopadhyay P, Rajesh M, Haskó G, Hawkins BJ, Madesh M, Pacher P (2007) Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2:2295–2301
Cande C, Vahsen N, Garrido C, Kroemer G (2004) Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ 11:591–595
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H (2015) Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33:1582–1614
Kim HR, Park CG, Jung JY (2014) Acacetin (5,7-dihydroxy-4′-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-kappaB/Akt signaling in prostate cancer cells. Int J Mol Med 33:317–324
Bhat TA, Nambiar D, Tailor D, Pal A, Agarwal R, Singh RP (2013) Acacetin inhibits in vitro and in vivo angiogenesis and downregulates Stat signaling and VEGF expression. Cancer Prev Res 6:1128–1139
Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, Lind GE, Lothe RA (2013) Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2:e71
Barnum KJ, O’Connell MJ (2014) Cell Cycle Regulation by Checkpoints. Methods Mol Biol 1170:29–40
Woo RA, Poon RY (2003) Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2:315–324
Wong NACS, Pignatelli M (2002) β-catenin—a linchpin in colorectal carcinogenesis? Am J Pathol 160:389–401
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44:479–496
Otera H, Ohsakaya S, Nagaura ZI, Ishihara N, Mihara K (2005) Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J 24:1375–1386
Acknowledgments
NP is recipient of UGC Non-NET Fellowship from Central University of Gujarat, Gandhinagar, UCSY is recipient of Ramanujan Fellowship from Department of Science and Technology (DST), Govt of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prasad, N., Sharma, J.R. & Yadav, U.C.S. Induction of growth cessation by acacetin via β-catenin pathway and apoptosis by apoptosis inducing factor activation in colorectal carcinoma cells. Mol Biol Rep 47, 987–1001 (2020). https://doi.org/10.1007/s11033-019-05191-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05191-x