Abstract
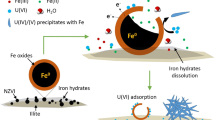
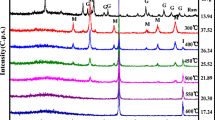
Montmorillonite-supported nanoscale zero-valent iron (M-nZVI) was synthesized by sodium borohydride reduction and characterized by X-ray diffraction, X-ray photoelectron spectroscopy, and field emission scanning electron microscopy (FE-SEM). The interaction of uranium with M-nZVI was studied using batch technique under different experimental conditions such as pH, ionic strength, initial U(VI) concentration, solid-to-liquid ration (m/V), and temperature. The presence of montmorillonite decreased the aggregation while increased the specific surface area (SSA) of the iron nanoparticles. The SSA for as-synthesized M-nZVI was 91.42 m2/g, higher than 26.60 and 10.23 m2/g for nZVI and montmorillonite, respectively. The removal efficiency of U(VI) using M-nZVI was significantly affected by the pH of the aqueous solution, whereas it was slightly affected by ionic strength and temperature. The isoelectric point of M-nZVI was at pH 5.6; however the results indicated that the optimum removal efficiency of U(VI) using M-nZVI was achieved at a pH range 3.0–5.0. The experiments with aqueous solution containing 100 μg/L of U(VI) showed that the removal efficiency of the as-synthesized M-nZVI was about 978 μg/g at pH 3.0. These results show that M-nZVI has a potential as a novel material for removing U(VI) from aqueous solution.








Similar content being viewed by others
References
Ren X, Wang S, Yang S, Li J (2009) J Radioanal Nucl Chem 283:253–259
Song X, Wang Y, Cai J, Lu S, Chen Y (2012) J Radioanal Nucl Chem 295:685–695
Zhu W, Liu Z, Chen L, Dong Y (2011) J Radioanal Nucl Chem 289:781–788
Singer DM, Maher K, Brown GE (2009) Geochim Cosmochim Acta 73:5989–6007
Singer DM, Chatman SM, Ilton ES, Rosso KM, Banfield JF, Waychunas GA (2012) Environ Sci Technol 46:3811–3820
Gao L, Yang Z, Shi K, Wang X, Guo Z, Wu W (2010) J Radioanal Nucl Chem 284:519–526
Akcay H (1998) J Radioanal Nucl Chem 237:133–137
Missana T, Garcia–Gutierrez M, Alonso U (2008) Phys Chem Earth 33:S156–S162
Aytas SO, Akyil S, Eral M (2003) J Radioanal Nucl Chem 260:119–125
Missana T, Garcia–Gutierrez M, Maffiotte C (2003) J Colloid Interface Sci 260:291–301
Singer DM, Chatman SM, Ilton ES, Rosso KM, Banfield JF, Waychunas GA (2012) Environ Sci Technol 46:3821–3830
Mellah A, Chegrouche S, Barkat M (2006) J Colloid Interface Sci 296:434–441
El Aamrani FZ, Duro L, de Pablo J, Bruno J (2002) Appl Geochem 17:399–408
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Bioresource Technol 96:1241–1248
Zhang A, Uchiyama G, Asakura T (2005) React Funct Polym 63:143–153
Sun YP, Li XQ, Cao J, Zhang WX, Wang HP (2006) Adv Colloid Interface Sci 120:47–56
Dickinson M, Scott TB (2010) J Hazard Mater 178:171–179
Riba O, Scott TB, Ragnarsdottir KV, Allen GC (2008) Geochim Cosmochim Acta 72:4047–4057
He F, Zhao D (2005) Environ Sci Technol 39:3314–3320
Uezuem C, Shahwan T, Eroglu AE, Hallam KR, Scott TB, Lieberwirth I (2009) Appl Clay Sci 43:172–181
Shi LN, Zhang X, Chen ZL (2011) Water Res 45:886–892
Li Z, Jones HK, Zhang P, Bowman RD (2007) Chemosphere 68:1861–1866
Wang W, Zhou MH, Mao Q, Yue JJ, Wang X (2010) Catal Commun 11:937–941
Zhang X, Lin S, Lu XQ, Chen ZL (2010) Chem Eng J 163:243–248
Su J, Lin S, Chen ZL, Megharaj M, Naidu R (2011) Desalination 280:167–173
Shahwan T, Uzum CC, Erouglu AE, Lieberwirth I (2010) Appl Clay Sci 47:257–262
Yuan P, Fan M, Yang D, He H, Liu D, Yuan A, Zhu J, Chen T (2009) J Hazard Mater 166:821–829
Evans N, Warwick P, Lewis T, Bryan N (2011) Environ Chem Lett 9:25–30
Chen ZX, ** XY, Chen ZL, Megharaj M, Naidu R (2011) J Colloid Interface Sci 363:601–607
Hu T, Tan L (2011) J Radioanal Nucl Chem 292:103–112
Kim SA, Kannan KS, Lee K, Park Y, Shea PJ, Lee W, Kim H, Oh B (2013) Chem Eng J 217:54–60
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Li, Y., **g, C. et al. Removal of uranium from aqueous solution using montmorillonite-supported nanoscale zero-valent iron. J Radioanal Nucl Chem 299, 329–336 (2014). https://doi.org/10.1007/s10967-013-2779-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2779-1