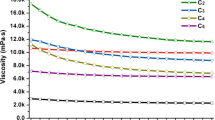
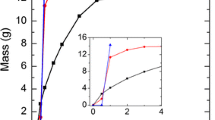
Abstract
A major obstacle for polymeric diphenylmethane diisocyanate (pMDI) share market growth in wood-particle and wood-fiber composites is its low adhesive tack property. In this work, we explore the improvement of pMDI tack by partial substitution using natural polymeric fractions derived from soybean hulls available at a relatively low cost. To that end, pectin was isolated from the soybean hull, and the remaining fibrous residue was fibrillated using mechanical treatment to produce both lignin-containing and chemically bleached cellulose nanofibrils (LCNFs and BCNFs, respectively). Pectin-isolated fractions from the soybean hull were chemically characterized. Pectin and nanofibrillated cellulose were used as additives in pMDI dispersions to improve its tack property. To determine the tack properties of the soybean hull-based-pMDI dispersions, rheological analysis was optimized based on an adaptation of the probe-tack test. Results clearly showed increased tack properties in pMDI/pectin/CNFs dispersions with the combination between soybean hull pectin and LCNFs exceeding the sum of their isolated effects compared to pMDI resin alone.





Similar content being viewed by others
References
Data, https://www.reportsanddata.com R and Methylene Diphenyl Diisocyanate (MDI) Market Size, 2019–2026. https://www.reportsanddata.com/report-detail/methylene-diphenyl-diisocyanate-mdi-market. Accessed 17 Aug 2020
Gao D, Fan B, Zhang B et al (2019) Storage stability of polyamidoamine-epichlorohydrin resin and its effect on the properties of defatted soybean flour-based adhesives. Int J Adhes Adhes 91:92–101. https://doi.org/10.1016/j.ijadhadh.2019.03.006
Asafu-Adjaye O, Via B, Banerjee S (2020) Increasing cold tack of polymeric methylene diphenyl diisocyanate resin with partial soy flour substitution. For Prod J 70:143–144. https://doi.org/10.13073/FPJ-D-19-00049
Frihart CR (2015) Introduction to special issue: wood adhesives: past present and future. Forest Prod J. 65:4–8. https://doi.org/10.13073/65.1-2.4
Chen H, Nair SS, Chauhan P, Yan N (2019) Lignin containing cellulose nanofibril application in pMDI wood adhesives for drastically improved gap-filling properties with robust bondline interfaces. Chem Eng J 360:393–401
Frihart C (2012) Wood adhesion and adhesives. In: Handbook of wood chemistry and wood composites, 2nd Ed. CRC Press, pp 255–320
Zosel A (1998) The effect of fibrilation on the tack of pressure sensitive adhesives. Int J Adhes Adhes 18:265–271. https://doi.org/10.1016/S0143-7496(98)80060-2
Wilson JB (1980) Isocyanate adhesives as binders for composition board. Wood adhesives-research, application, and needs Madison: USDA Forest Service (Forest Products Laboratory). pp. 117–121
Higgins ED (1995) Isocyanate reactive hot-melt adhesive for veneer laminates. For Prod J 45:72
Asafu-Adjaye O, Via B, Banerjee S (2020) Increasing cold tack of polymeric methylene diphenyl diisocyanate resin with partial soy flour substitution. For Prod J 70(1):143-144
Asafu-Adjaye O (2020) Wood composites production with epoxy resin substituted liquefied lignocellulosic biomass, and polymeric Diphenylmethane Diisocyanate substituted defatted soy flour as wood adhesives
Berk Z (1992) Technology of production of edible flours and protein products from soybeans.
Kumar Singh A, Singh Mehra D, Kumar Niyogi U et al (2012) Effect of tackifier and crosslinkers on electron beam curable polyurethane pressure sensitive adhesive. Radiat Phys Chem 81:547–552. https://doi.org/10.1016/j.radphyschem.2012.01.017
Shimizu K, Phanopoulos C, Loenders R et al (2010) The characterization of the interfacial interaction between polymeric methylene diphenyl diisocyanate and aluminum: a ToF-SIMS and XPS study. Surf Interface Anal 42:1432–1444. https://doi.org/10.1002/sia.3586
Dillingham RG, Moriarty C (2003) The adhesion of isocyanate-based polymers to steel. J Adhes 79:269–285
Yuan J, Shi SQ (2009) Effect of the addition of wood flours on the properties of rigid polyurethane foam. J Appl Polym Sci 113:2902–2909. https://doi.org/10.1002/app.30322
Hosseinpourpia R, Adamopoulos S, Mai C et al (2019) Properties of medium-density fibreboards bonded with dextrin-based wood adhesive. Wood Res 64:10
Ivdre A, Cabulis U, Arshanitsa A (2014) Wheat straw lignin as filler for rigid polyurethane foams on the basis of tall oil amide. Polimery 59:477–481. https://doi.org/10.14314/polimery.2014.477
Wysocka K, Szymona K, McDonald AG, Mamiński MŁ (2016) Characterization of thermal and mechanical properties of lignosulfonate- and Hydrolyzed lignosulfonate-based polyurethane foams. BioResources 11:7355–7364
Zhang J, Hori N, Takemura A (2021) Reinforcement of agricultural wastes liquefied polyols based polyurethane foams by agricultural wastes particles. J Appl Polym Sci 138:50583. https://doi.org/10.1002/app.50583
Hornus M, Via BK, Gallagher T, Peresin MS (2021) Partial substitution of pMDI with lignin containing cellulose nanofibrils: low density oriented strand board. Wood Mat Sci Eng 16:391–396. https://doi.org/10.1080/17480272.2020.1769722
Sonnenschein MF, Wendt BL, Sonnenschein GF (2005) Interfacial factors affecting polymeric diphenylmethane diisocyanate/wood bond strength. J Appl Polym Sci 98:449–455. https://doi.org/10.1002/app.21946
FAOSTAT. http://www.fao.org/faostat/en/#search/Soybeans. Accessed 16 Feb 2021
Hult E-L, Iotti M, Lenes M (2010) Efficient approach to high barrier packaging using microfibrillar cellulose and shellac. Cellulose 17:575–586. https://doi.org/10.1007/s10570-010-9408-8
Gnanasambandam R, Proctor A (1999) Preparation of soy hull pectin. Food Chem 65:461–467. https://doi.org/10.1016/S0308-8146(98)00197-6
Cassales A, de Souza-Cruz PB, Rech R, Záchia Ayub MA (2011) Optimization of soybean hull acid hydrolysis and its characterization as a potential substrate for bioprocessing. Biomass Bioenerg 35:4675–4683. https://doi.org/10.1016/j.biombioe.2011.09.021
Kalapathy U, Proctor A (2001) Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem 73:393–396
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11:266–277. https://doi.org/10.1016/j.pbi.2008.03.006
Harholt J, Suttangkakul A, Scheller HV (2010) Biosynthesis of pectin. Plant Physiol 153:384–395
Rojo E, Soledad Peresin M, Sampson W et al (2015) Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem 17:1853–1866. https://doi.org/10.1039/C4GC02398F
Chen H, Gnanasekar P, Nair SS et al (2020) Lignin as a key component in lignin-containing cellulose nanofibrils for enhancing the performance of polymeric diphenylmethane diisocyanate wood adhesives. ACS Sustainable Chem Eng 8:17165–17176. https://doi.org/10.1021/acssuschemeng.0c05642
Iglesias MC, Shivyari N, Norris A et al (2020) The effect of residual lignin on the rheological properties of cellulose nanofibril suspensions. J Wood Chem Technol 40:370–381. https://doi.org/10.1080/02773813.2020.1828472
Diop CIK, Tajvidi M, Bilodeau MA et al (2017) Evaluation of the incorporation of lignocellulose nanofibrils as sustainable adhesive replacement in medium density fiberboards. Ind Crops Prod 109:27–36
Hubbe MA, Tayeb P, Joyce M et al (2017) Rheology of nanocellulose-rich aqueous suspensions: a review. BioResources 12:9556–9661
Iglesias MC, Hamade F, Aksoy B et al (2021) Correlations between rheological behavior and intrinsic properties of nanofibrillated cellulose from wood and soybean hulls with varying lignin content. BioResources 16:4831–4845. https://doi.org/10.15376/biores.16.3.4831-4845
Dufresne A (2013) Nanocellulose: a new ageless bionanomaterial. Mater Today 16:220–227. https://doi.org/10.1016/j.mattod.2013.06.004
Jarvis MC (1984) Structure and properties of pectin gels in plant cell walls. Plant, Cell Environ 7:153–164. https://doi.org/10.1111/1365-3040.ep11614586
Fishman ML, Chau HK, Qi PX et al (2015) Characterization of the global structure of low methoxyl pectin in solution. Food Hydrocolloids 46:153–159
Technology of production of edible flours and protein products from soybeans. Chapter 4. https://www.fao.org/3/t0532e/t0532e05.htm. Accessed 30 Nov 2021
Dei HK (2011) Soybean as a feed ingredient for livestock and poultry. In: Recent trends for enhancing the diversity and quality of soybean products. IntechOpen
Zainudin BH, Wong TW, Hamdan H (2018) Design of low molecular weight pectin and its nanoparticles through combination treatment of pectin by microwave and inorganic salts. Polym Degrad Stab 147:35–40
Melton LD, Smith BG (2001) Determination of the uronic acid content of plant cell walls using a colorimetric assay. In: Current protocols in food analytical chemistry. WileyPublication
Monsoor MA, Proctor A (2001) Preparation and functional properties of soy hull pectin. J Am Oil Chem Soc 78:709
Monsoor MA (2005) Effect of drying methods on the functional properties of soy hull pectin. Carbohyd Polym 61:362–367
Monsoor MA (1981) Food chemicals codex, 3rd edn. The National Academies Press, Washington, DC
Hosseini SS, Khodaiyan F, Yarmand MS (2016) Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohyd Polym 140:59–65
de Sato MF, Rigoni DC, Canteri MHG et al (2011) Chemical and instrumental characterization of pectin from dried pomace of eleven apple cultivars. Acta Scientiarum Agronomy 33:383–389
Faravash RS, Ashtiani FZ (2008) The influence of acid volume, ethanol-to-extract ratio and acid-washing time on the yield of pectic substances extraction from peach pomace. Food Hydrocolloids 22:196–202
Gnanasambandam R, Proctor A (2000) Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem 68:327–332
Monsoor MA, Kalapathy U, Proctor A (2001) Improved method for determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. J Agric Food Chem 49:2756–2760
Espino E, Cakir M, Domenek S et al (2014) Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind Crops Prod 62:552–559
Hernández JA, Romero VH, Escalante A et al (2018) Agave tequilana bagasse as source of cellulose nanocrystals via organosolv treatment. BioResources 13:3603–3614
Iglesias MC (2018) Lignin-containing cellulose nanofibrils (LCNF): processing and characterization. Master’s thesis, 2018, Auburn University. http://hdl.handle.net/10415/6303
Villada Y, Iglesias MC, Casis N et al (2018) Cellulose nanofibrils as a replacement for xanthan gum (XGD) in water based muds (WBMs) to be used in shale formations. Cellulose 25:7091–7112
Stana-Kleinschek K, Strnad S, Ribitsch V (1999) Surface characterization and adsorption abilities of cellulose fibers. Polym Eng Sci 39:1412–1424. https://doi.org/10.1002/pen.11532
Espinosa E, Tarrés Q, Delgado-Aguilar M et al (2016) Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 23:837–852. https://doi.org/10.1007/s10570-015-0807-8
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Park S, Baker JO, Himmel ME et al (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10. https://doi.org/10.1186/1754-6834-3-10
Team Rs (2020) RStudio: integrated development for R. RStudio, PBC, Boston
Schultz BB (1985) Levene’s test for relative variation. Syst Biol 34:449–456. https://doi.org/10.1093/sysbio/34.4.449
Hsu HP (1997) Schaum’s outline of theory and problems of probability, random variables, and random processes. McGraw-Hill, New York
Corredor DY, Sun XS, Salazar JM et al (2008) Enzymatic hydrolysis of soybean hulls using dilute acid and modified steam-explosion pretreatments. J Biobased Mater Bioenergy 2:43–50
Mielenz JR, Bardsley JS, Wyman CE (2009) Fermentation of soybean hulls to ethanol while preserving protein value. Biores Technol 100:3532–3539
Blasi D, Titgemeyer E, Drouillard J, Paisley S, Brouk, M (2000) Soybean hulls, composition and feeding value for beef and dairy cattle. Kansas State: University Agricultural Experimental Station and Cooperative Extension Service. Bull. MF-2438. https://bookstore.ksre.ksu.edu/pubs/mf2438.pdf
Liu H-M, Li H-Y (2017) Application and conversion of soybean hulls. Soybean - The Basis Yield Bio Prod. https://doi.org/10.5772/66744
Qing Q, Guo Q, Zhou L et al (2017) Comparison of alkaline and acid pretreatments for enzymatic hydrolysis of soybean hull and soybean straw to produce fermentable sugars. Ind Crops Prod 109:391–397. https://doi.org/10.1016/j.indcrop.2017.08.051
Porfiri MC, Wagner JR (2018) Extraction and characterization of soy hull polysaccharide-protein fractions. Analysis of aggregation and surface rheology. Food Hydrocolloids 79:40–47. https://doi.org/10.1016/j.foodhyd.2017.11.050
Nakamura A, Furuta H, Maeda H et al (2002) Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci Biotechnol Biochem 66:1301–1313. https://doi.org/10.1271/bbb.66.1301
Porfiri MC, Cabezas DM, Wagner JR (2016) Comparative study of emulsifying properties in acidic condition of soluble polysaccharides fractions obtained from soy hull and defatted soy flour. J Food Sci Technol 53:956–967
Rolin C, De Vries J (1990) Pectin. In: Harris P (ed) Food gels. Unilever Research Laboratory, Bedford, UK
Axelos M, Thibault J (1991) The chemistry of low-methoxyl pectin gelation. Chem Technol Pectin 6:109–108
Oakenfull D, Scott A (1984) Hydrophobic interaction in the gelation of high methoxyl pectins. J Food Sci 49:1093–1098. https://doi.org/10.1111/j.1365-2621.1984.tb10401.x
Durand D, Bertrand C, Clark AH, Lips A (1990) Calcium-induced gelation of low methoxy pectin solutions—Thermodynamic and rheological considerations. Int J Biol Macromol 12:14–18
Kastner H, Einhorn-Stoll U, Senge B (2012) Structure formation in sugar containing pectin gels – Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocolloids 27:42–49. https://doi.org/10.1016/j.foodhyd.2011.09.001
Garnier C, Axelos M, Thibault J-F (1991) Dynamic viscoelasticity and thermal behaviour of pectin—calcium gels. Food Hydrocolloids 5:105–108
Zhang L, Ye X, Ding T et al (2013) Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason Sonochem 20:222–231. https://doi.org/10.1016/j.ultsonch.2012.07.021
Wang S, Zhao L, Li Q et al (2019) Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocolloids 91:34–39. https://doi.org/10.1016/j.foodhyd.2018.12.054
Júnior JAA, Baldo JB (2014) The behavior of zeta potential of silica suspensions. New J Glass Ceramics 04:29. https://doi.org/10.4236/njgc.2014.42004
Reischl M, Stana-Kleinschek K, Ribitsch V (2006) Electrokinetic investigations of oriented cellulose polymers. Macromol Symp 244:31–47. https://doi.org/10.1002/masy.200651203
Hai LV, Kim HC, Kafy A et al (2017) Green all-cellulose nanocomposites made with cellulose nanofibers reinforced in dissolved cellulose matrix without heat treatment. Cellulose 24:3301–3311. https://doi.org/10.1007/s10570-017-1333-7
Galiwango E, Abdel Rahman NS, Al-Marzouqi AH et al (2019) Isolation and characterization of cellulose and α-cellulose from date palm biomass waste. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e02937
Palacios Hinestroza H, Hernández Diaz JA, Esquivel Alfaro M et al (2019) Isolation and characterization of nanofibrillar cellulose from agave tequilana weber bagasse. Adv Mater Sci Eng 2019:1342547. https://doi.org/10.1155/2019/1342547
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues–wheat straw and soy hulls. Biores Technol 99:1664–1671
Huang Y, Wang Z, Wang L et al (2016) Analysis of lignin aromatic structure in wood fractions based on IR spectroscopy. J Wood Chem Technol 36:377–382
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Morán JI, Alvarez VA, Cyras VP, Vázquez A (2008) Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 15:149–159. https://doi.org/10.1007/s10570-007-9145-9
ASTM International. Standard test methods for loop tack. (ASTM D6195-03). https://www.astm.org/d6195-03r19.html
ASTM International. Standard test method for pressure-sensitive tack of adhesives using an inverted probe machine. (ASTM-D2979-01). https://www.astm.org/d2979-16.html
ASTM International. Standard test method for tack of pressure-sensitive adhesives by rolling ball. (ASTM D3121-17). https://www.astm.org/d3121-17.html
Tirumkudulu M, Russel WB, Huang TJ (2003) On the measurement of “tack” for adhesives. Phys Fluids 15:1588–1605. https://doi.org/10.1063/1.1571058
Tirumkudulu M, Russel WB, Huang TJ (2003) Measuring the “tack” of waterborne adhesives. J Rheol 47:1399–1415. https://doi.org/10.1122/1.1608953
Wang X (2016) Filler effects in resole adhesive formulations. Thesis, Virginia Tech
Adams MA, Conway TL (2014) Eta squared. In: Michalos AC (ed) Encyclopedia of quality of life and well-being research. Springer, Netherlands, Dordrecht, pp 1965–1966
Acknowledgements
This work was done under the Project Grant 1940-352-0701-D from the United Soybean Board (USB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Handling Editor: Stephen Eichhorn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hernandez, J.A., Soni, B., Iglesias, M.C. et al. Soybean hull pectin and nanocellulose: tack properties in aqueous pMDI dispersions. J Mater Sci 57, 5022–5035 (2022). https://doi.org/10.1007/s10853-022-06938-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-06938-x