Abstract
Purpose
Body composition parameters including muscle and adipose tissue measurements have emerged as prognostic factors in cancer patients. Besides cell cycle regulation, CDK 4 and 6 also control metabolic processes (lipid synthesis, glycolysis, and mitochondrial function). We studied the impact of baseline body composition parameters on response to CDK 4/6 inhibition and changes on body composition during treatment.
Methods
Retrospective study of 50 patients treated at Institut Jules Bordet between December 2016 and August 2019 with endocrine therapy and CDK 4/6 inhibitor as first or second-line treatment for metastatic breast cancer (BC). CT-based body composition analysis was performed at 3 time points. Cox regression and Kaplan–Meier method were used for the association with Progression-free survival (PFS). Changes in body composition parameters were described in means and compared using paired sampled T test.
Results
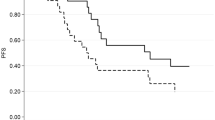
Baseline sarcopenia was present in 40% of patients and associated with a significantly worse PFS compared to patients without sarcopenia (20.8 vs 9.6 months, HR 2.52; 95% CI 1.02–6.19, p = 0.037). Patients with higher visceral fat index and higher visceral fat density had better PFS (20.8 vs 10.4 months, HR 0.40; 95% CI 0.16–0.99 p = 0.041-stratified for treatment line). No significant alterations in body composition parameters during treatment were observed.
Conclusion
Sarcopenia is a potential early marker of poor prognosis among patients with metastatic BC treated with CDK 4/6 inhibitors. CT scan evaluation of sarcopenia and adiposity revealed significant prognostic information. Visceral fat could also play an important role in response to CDK 4/6 inhibitors, deserving further investigation.




Similar content being viewed by others
References
Trestini I, Carbognin L, Monteverdi S et al (2018) Clinical implication of changes in body composition and weight in patients with early-stage and metastatic breast cancer. Crit Rev Oncol Hematol 129:54–66
Ligibel JA, Alfano CM, Courneya KS et al (2014) American Society of Clinical Oncology Position Statement on Obesity and Cancer. J Clin Oncol 32:3568–3574
Cho WK, Choi DH, Park W et al (2018) Effect of Body Mass Index on Survival in Breast Cancer Patients According to Subtype, Metabolic Syndrome, and Treatment. Clinical Breast Cancer 18:e1141–e1147
Chan DSM, Vieira AR, Aune D et al (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25:1901–1914
Yerushalmi R, Dong B, Chapman JW et al (2017) Impact of baseline BMI and weight change in CCTG adjuvant breast cancer trials. Ann Oncol 28:1560–1568
Zewenghiel L, Lindman H, Valachis A (2018) Impact of body mass index on the efficacy of endocrine therapy in patients with metastatic breast cancer - A retrospective two-center cohort study. The Breast 40:136–140
Charette N, Vandeputte C, Ameye L et al (2019) Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: a post hoc analysis of two non-randomized phase II trials. BMC Cancer 19:134
Greenlee H, Unger JM, LeBlanc M et al (2017) Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol Biomarkers Prev 26:21–29
Caan BJ, Feliciano EMC, Kroenke CH (2018) The Importance of Body Composition in Explaining the Overweight Paradox in Cancer—Counterpoint. Cancer Res 78:1906–1912
Brown JC, Cespedes Feliciano EM, Caan BJ (2018) The evolution of body composition in oncology—epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle 9:1200–1208
Goetz MP, Toi M, Campone M et al (2017) MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 35:3638–3646
Hortobagyi GN, Stemmer SM, Burris HA et al (2018) Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 29:1541–1547
Rugo HS, Finn RS, Diéras V et al (2019) Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 174:719–729
Aguilar V, Fajas L (2010) Cycling through metabolism. EMBO Mol Med 2:338–348
Lagarrigue S, Lopez-Mejia IC, Denechaud P-D et al (2015) CDK4 is an essential insulin effector in adipocytes. Journal of Clinical Investigation 126:335–348
Fajas L (2013) Re-thinking cell cycle regulators: the cross-talk with metabolism. Frontiers in Oncology 3:1–6
Hou X, Zhang Y, Li W et al (2018) CDK6 inhibits white to beige fat transition by suppressing RUNX1. Nature Communications 9:1023
Iqbal NJ, Lu Z, Liu SM et al (2018) Cyclin-dependent kinase 4 is a preclinical target for diet-induced obesity. JCI Insight 3:e123000
Thibault R, Genton L, Pichard C (2012) Body composition: why, when and for who? Clin Nutr 31:435–447
Mourtzakis M, Prado CMM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Caan BJ, Cespedes Feliciano EM, Prado CM et al (2018) Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol 4:798–804
Rier HN, Jager A, Sleijfer S et al (2018) Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 168:95–105
Rier HN, Jager A, Sleijfer S et al (2016) The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist 21:1396–1409
Yip C, Dinkel C, Mahajan A et al (2015) Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 6:489–497
Kazemi-Bajestani SMR, Mazurak VC, Baracos V (2016) Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 54:2–10
Huang D-D, Wang S-L, Zhuang C-L et al (2015) Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis 17:O256–264
Malietzis G, Johns N, Al-Hassi HO et al (2016) Low Muscularity and Myosteatosis Is Related to the Host Systemic Inflammatory Response in Patients Undergoing Surgery for Colorectal Cancer. Ann Surg 263:320–325
Prado CMM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
Prado CMM, Baracos VE, McCargar LJ et al (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13:3264–3268
Rossi F, Valdora F, Bignotti B et al (2019) Evaluation of body Computed Tomography-determined sarcopenia in breast cancer patients and clinical outcomes: A systematic review. Cancer Treat Res Commun 21:100154
Villaseñor A, Ballard-Barbash R, Baumgartner K et al (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv 6:398–406
Rier HN, Jager A, Sleijfer S et al (2017) Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast 31:9–15
Prado CMM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Gevorgyan A, Bregni G, Galli G et al (2016) Body mass index and clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer. Tumori 102:e11–14
Pizzuti L, Natoli C, Gamucci T et al (2017) Anthropometric, clinical and molecular determinants of treatment outcomes in postmenopausal, hormone receptor positive metastatic breast cancer patients treated with fulvestrant: Results from a real word setting. Oncotarget 8:69025–69037
Ioannides SJ, Barlow PL, Elwood JM et al (2014) Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: a systematic review. Breast Cancer Res Treat 147:237–248
Miard S, Fajas L (2005) Atypical transcriptional regulators and cofactors of PPARgamma. Int J Obes (Lond) 29(Suppl 1):S10–12
Abella A, Dubus P, Malumbres M et al (2005) Cdk4 promotes adipogenesis through PPARgamma activation. Cell Metab 2:239–249
Fielding RA, Vellas B, Evans WJ, et al: Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12:249–256, 2011
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Funding
This study has received no funding.
Author information
Authors and Affiliations
Contributions
MA and CV: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, writing original draft and writing-review & editing. DE, RC, CDA and AH: Investigation, Validation, Visualization, writing original draft and writing review & editing. MB: Formal analysis, Investigation, Methodoloy, Sotware, Validation, Visualization, writing original draft and writing review & editing. AA and MP: Resources, Project administration, Supervision, Writing – review & editing. EA: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Supervision, Project administration, writing- original draft and writing-review & editing. All authors have read and approved this manuscript and ensure this submission is not under consideration elsewhere.
Corresponding author
Ethics declarations
Conflict of interest
MAF, CV, AH and CDA: none. DE: Funding for his ESMO fellowship: Novartis. RC: speaker honoraria from Boehringer Ingelheim, AstraZeneca and Janssen, travel grants from AstraZeneca and Pfizer. MB: travel grant and speaker honoraria from Roche/GNE. AA: Advisory role, speaker fees and research funding for his institute from: Roche, Lilly, Amgen, EISAI, BMS, Pfizer, Novartis, MSD, Genomic Health, Ipsen, AstraZeneca, Bayer, Leo Pharma. M.P.: Board Member (Scientific Board): Oncolytics, Radius; Consultant (honoraria): AstraZeneca, Camel-IDS, Crescendo Biologics, Debiopharm, G1 Therapeutics, Genentech, Huya, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Oncolytics, Periphagen, Pfizer, Roche, Seattle Genetics; Research grants to her Institute: AstraZeneca, Lilly, MSD, Novartis, Pfizer, Radius, Roche-Genentech, Servier, Synthon; Speakers bureau/stock ownership: none. EA: honoraria and advisory board: Roche/GNE, Novartis, Seatle Genetics; travel grants: Roche/GNE, GSK/Novartis; co-principal investigator of the LORELEI trial (NCT02273973). Research grant for his institute: Roche/GNE, Astra-Zeneca, Novartis, and Servier.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.This study was approved by an internal ethics committee (EC3048), and informed consent was not required as patient’s information was retrieved from retrospective review of medical records only. The identity of the patients was kept always anonymously.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Franzoi, M.A., Vandeputte, C., Eiger, D. et al. Computed tomography-based analyses of baseline body composition parameters and changes in breast cancer patients under treatment with CDK 4/6 inhibitors. Breast Cancer Res Treat 181, 199–209 (2020). https://doi.org/10.1007/s10549-020-05617-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05617-2