Abstract
Purpose
We aimed to investigate whether visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and skeletal muscle area (SMA) index are predictive for efficacy and hematological toxicity in ER + HER2—metastatic breast cancer (BC) patients who received CDK 4/6 inhibitors.
Methods
This retrospective cohort study analyzed 52 patients who were treated with CDK 4/6 inhibitors between January 2018 and February 2021. The values of VAT, SAT, SMA indices and hematological parameters were noted before the start, at the third and sixth months of this treatment. The skeletal muscle area (SMA) and adipose tissue measurements were calculated at the level of the third lumbar vertebra. A SMA-index value of <40 cm2/m2 was accepted as the threshold value for sarcopenia.
Results
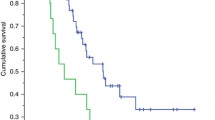
Patients with sarcopenia had a worse progression-free survival (PFS) compared to patients without sarcopenia (19.6 vs. 9.0 months, p = 0.005). Patients with a high-VAT-index had a better PFS (20.4 vs. 9.3 months, p = 0.033). Only the baseline low-SMA- index (HR: 3.89; 95% CI: 1.35–11.25, p = 0.012) and baseline low-VAT-index (HR: 2.15; 95% CI: 1.02–4.53, p = 0.042) had significantly related to poor PFS in univariate analyses. The low-SMA-index was the only independent factor associated with poor PFS (HR: 3.99; 95% CI: 1.38–11.54, p = 0.011). No relationship was observed between body composition parameters and grade 3–4 hematological toxicity.
Conclusion
The present study supported the significance of sarcopenia and low visceral adipose tissue as potential early indicators of poor PFS in patients treated with CDK 4/6 inhibitors.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [KBY], upon reasonable request.
References
Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26:861–868. https://doi.org/10.1007/s00520-017-3902-6
Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS, Qin LQ (2016) Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev 17:1167–1177. https://doi.org/10.1111/obr.12443
Yerushalmi R, Dong B, Chapman JW, Goss PE, Pollak MN, Burnell MJ, Levine MN, Bramwell VHC, Pritchard KI, Whelan TJ, Ingle JN, Shepherd LE, Parulekar WR, Han L, Ding K, Gelmon KA (2017) Impact of baseline BMI and weight change in CCTG adjuvant breast cancer trials. Ann Oncol 28:1560–1568. https://doi.org/10.1093/annonc/mdx152
Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25:1901–1914. https://doi.org/10.1093/annonc/mdu042
Cho WK, Choi DH, Park W, Cha H, Nam SJ, Kim SW, Lee JE, Yu J, Im YH, Ahn JS, Park YH, Kim JY, Ahn S (2018) Effect of body mass index on survival in breast cancer patients according to subtype, metabolic syndrome, and treatment. Clin Breast Cancer 18:e1141–e1147. https://doi.org/10.1016/j.clbc.2018.04.010
Zewenghiel L, Lindman H, Valachis A (2018) Impact of body mass index on the efficacy of endocrine therapy in patients with metastatic breast cancer-a retrospective two-center cohort study. Breast 40:136–140. https://doi.org/10.1016/j.breast.2018.05.005
Trestini I, Carbognin L, Monteverdi S, Zanelli S, De Toma A, Bonaiuto C, Nortilli R, Fiorio E, Pilotto S, Di Maio M, Gasbarrini A, Scambia G, Tortora G, Bria E (2018) Clinical implication of changes in body composition and weight in patients with early-stage and metastatic breast cancer. Crit Rev Oncol Hematol 129:54–66. https://doi.org/10.1016/j.critrevonc.2018.06.011
Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, Petrakova K, Valeria Bianchi G, Martín M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Ji Y, Wang C, Deore U, Chakravartty A, Zarate JP, Taran T, Fasching PA (2021) Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol 32:1015–1024. https://doi.org/10.1016/j.annonc.2021.05.353
Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, Frenzel M, Hardebeck MC, Cox J, Barriga S, Toi M, Iwata H, Goetz MP (2019) MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5:5. https://doi.org/10.1038/s41523-018-0097-z
Hanse EA, Mashek DG, Becker JR, Solmonson AD, Mullany LK, Mashek MT, Towle HC, Chau AT, Albrecht JH (2012) Cyclin D1 inhibits hepatic lipogenesis via repression of carbohydrate response element binding protein and hepatocyte nuclear factor 4α. Cell Cycle 11:2681–2690. https://doi.org/10.4161/cc.21019
Iqbal NJ, Lu Z, Liu SM, Schwartz GJ, Chua S Jr, Zhu L (2018) Cyclin-dependent kinase 4 is a preclinical target for diet-induced obesity. JCI insight 3:e123000. https://doi.org/10.1172/jci.insight.123000
Hou X, Zhang Y, Li W, Hu AJ, Luo C, Zhou W, Hu JK, Daniele SG, Wang J, Sheng J, Fan Y, Greenberg AS, Farmer SR, Hu MG (2018) CDK6 inhibits white to beige fat transition by suppressing RUNX1. Nat Commun 9:1023. https://doi.org/10.1038/s41467-018-03451-1
Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, **ao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan ML, Prado CM (2017) Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS study). Cancer Epidemiol Biomarkers Prev 26:1008–1015. https://doi.org/10.1158/1055-9965.EPI-17-0200
Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJ, Goh V (2015) Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 6:489–497. https://doi.org/10.1007/s13244-015-0414-0
Thibault R, Genton L, Pichard C (2012) Body composition: why, when and for who? Clin Nutr 31:435–447. https://doi.org/10.1016/j.clnu.2011.12.011
Caan BJ, Cespedes Feliciano EM, Kroenke CH (2018) The importance of body composition in explaining the overweight paradox in cancer—counterpoint. Cancer Res 78:1906–1912. https://doi.org/10.1158/0008-5472.CAN-17-3287
Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC (2018) Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 8:11369. https://doi.org/10.1038/s41598-018-29825-5
Vangelov B, Bauer J, Kotevski D, Smee RI (2022) The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr 127:722–735. https://doi.org/10.1017/S0007114521001446
Bahat G, Turkmen BO, Aliyev S, Catikkas NM, Bakir B, Karan MA (2021) Cut-off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin Nutr 40:4360–4365. https://doi.org/10.1016/j.clnu.2021.01.010
Chindapasirt J (2015) Sarcopenia in cancer patients. Asian Pac J Cancer Prev 16:8075–8077. https://doi.org/10.7314/apjcp.2015.16.18.8075
Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 37:1101–1113. https://doi.org/10.1016/j.clnu.2017.07.010
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926. https://doi.org/10.1158/1078-0432.CCR-08-2242
Deluche E, Lachatre D, Di Palma M, Simon H, Tissot V, Vansteene D, Meingan P, Mohebi A, Lenczner G, Pigneur F, Goldwasser F, Raynard B; SCAN Study Group (2022) Is sarcopenia a missed factor in the management of patients with metastatic breast cancer? Breast 61:84–90. https://doi.org/10.1016/j.breast.2021.12.014
Çağlayan D, Kocak MZ, Geredeli C, Tatli AM, Karakurt Eryılmaz M, Göksu SS, Araz M, Artaç M (2022) The effect of BMI on the outcomes of CDK 4/6 inhibitor therapy in HR-positive metastatic breast cancer patients. J Clin Oncol 40:e13010. https://doi.org/10.1200/JCO.2022.40.16_suppl.e13010
Artac M, Cağlayan D, Koçak M, Geredeli C, Tatli A, Goksu SS, Eryılmaz MK, Araz M (2022) The impact of body mass index (BMI) on the progression-free survival of CDK4/6 inhibitors in metastatic breast cancer patients (MBC). Ann Oncol 33:S644–S645. https://doi.org/10.1016/j.annonc.2022.07.274
Franzoi MA, Eiger D, Ameye L, Ponde N, Caparica R, De Angelis C, Brandão M, Desmedt C, Di Cosimo S, Kotecki N, Lambertini M, Awada A, Piccart M, Azambuja E (2021) Clinical implications of body mass index in metastatic breast cancer patients treated with abemaciclib and endocrine therapy. J Nat Cancer Inst 113:462–470. https://doi.org/10.1093/jnci/djaa116
Patel R, Li Z, Zimmerman BS, Fink MY, Wells JD, Zhou X, Ayers K, Redfern A, Newman S, Schadt E, Oh WK, Chen R, Tiersten A (2022) Impact of body mass index on the efficacy of aromatase inhibitors in patients with metastatic breast cancer. Breast Cancer Res Treat 192:313–319. https://doi.org/10.1007/s10549-021-06504-0
Franzoi MA, Vandeputte C, Eiger D, Caparica R, Brandão M, De Angelis C, Hendlisz A, Awada A, Piccart M, de Azambuja E (2020) Computed tomography-based analyses of baseline body composition parameters and changes in breast cancer patients under treatment with CDK 4/6 inhibitors. Breast Cancer Res Treat 181:199–209. https://doi.org/10.1007/s10549-020-05617-2
Kripa E, Rizzo V, Galati F, Moffa G, Cicciarelli F, Catalano C, Pediconi F (2022) Do body composition parameters correlate with response to targeted therapy in ER+/HER2- metastatic breast cancer patients? Role of sarcopenia and obesity. Front Oncol 12:987012. https://doi.org/10.3389/fonc.2022.987012
Park HJ, Shin Y, Park J, Kim H, Lee IS, Seo DW, Huh J, Lee TY, Park T, Lee J, Kim KW (2020) Development and validation of a deep learning system for segmentation of abdominal muscle and fat on computed tomography. Korean J Radiol 21:88–100. https://doi.org/10.3348/kjr.2019.0470
Park J, Gil JR, Shin Y, Won SE, Huh J, You MW, Park HJ, Sung YS, Kim KW (2019) Reliable and robust method for abdominal muscle mass quantification using CT/MRI: an explorative study in healthy subjects. PLoS ONE 14:e0222042. https://doi.org/10.1371/journal.pone.0222042
Murray TE, Williams D, Lee MJ (2017) Osteoporosis, obesity, and sarcopenia on abdominal CT: a review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom Radiol (NY) 42:2376–2386. https://doi.org/10.1007/s00261-017-1124-5
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259. https://doi.org/10.1158/1078-0432.CCR-04-0713
Park J, Morley TS, Kim M, Clegg DJ, Scherer PE (2014) Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10:455–465. https://doi.org/10.1038/nrendo.2014.94
Park J, Euhus DM, Scherer PE (2011) Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev 32:550–570. https://doi.org/10.1210/er.2010-0030
Iwase T, Sangai T, Nagashima T, Sakakibara M, Sakakibara J, Hayama S, Ishigami E, Masuda T, Miyazaki M (2016) Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med 5:41–48. https://doi.org/10.1002/cam4.571
Schapira DV, Clark RA, Wolff PA, Jarrett AR, Kumar NB, Aziz NM (1994) Visceral obesity and breast cancer risk. Cancer 74:632–639. https://doi.org/10.1002/1097-0142(19940715)74:2%3c632::aid-cncr2820740215%3e3.0.co;2-t
Kwon MR, Ko ES, Park MS, Jeong WK, Hwang NY, Kim JH, Lee JE, Kim SW, Yu JH, Han BK, Ko EY, Choi JS, Park KW (2022) Impact of skeletal muscle loss and visceral obesity measured using serial CT on the prognosis of operable breast cancers in asian patients. Korean J Radiol 23:159–171. https://doi.org/10.3348/kjr.2020.1475
Ritter A, Friemel A, Fornoff F, Adjan M, Solbach C, Yuan J, Louwen F (2015) Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget 6:34475–34493. https://doi.org/10.18632/oncotarget.5922
Donohoe CL, Doyle SL, Reynolds JV (2011) Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr 3:1–13. https://doi.org/10.1186/1758-5996-3-12
Van Kruijsdijk RC, Van Der Wall E, Visseren FL (2009) Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 18:2569–2578. https://doi.org/10.1158/1055-9965.EPI-09-0372
Bousquenaud M, Fico F, Solinas G, Rüegg C, Santamaria-Martínez A (2018) Obesity promotes the expansion of metastasis-initiating cells in breast cancer. Breast Cancer Res 20:104. https://doi.org/10.1186/s13058-018-1029-4
Osman MA, Hennessy BT (2015) Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin Med Insights Oncol 9:105–112. https://doi.org/10.4137/CMO.S32812
Gevorgyan A, Bregni G, Galli G, Ganzinelli M, Martinetti A, Lo Vullo S, Mariani L, Festinese F, Sottotetti E, de Braud F, Di Cosimo S (2016) Body mass index and clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer. Tumori 102:e11–e14. https://doi.org/10.5301/tj.5000515
Pizzuti L, Natoli C, Gamucci T, Mauri M, Sergi D, Di Lauro L, Paoletti G, Ruggeri E, Iezzi L, Sperduti I, Mentuccia L, Fabbri A, Maugeri-Saccà M, Moscetti L, Barba M, Vici P (2017) Anthropometric, clinical and molecular determinants of treatment outcomes in postmenopausal, hormone receptor positive metastatic breast cancer patients treated with fulvestrant: results from a real word setting. Oncotarget 8:69025–69037. https://doi.org/10.18632/oncotarget.16982
Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MCJM, Levin MD (2018) Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 168:95–105. https://doi.org/10.1007/s10549-017-4574-0
Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A (2021) Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs 81:317–331. https://doi.org/10.1007/s40265-020-01461-2
Petrelli F, Ghidini A, Pedersini R, Cabiddu M, Borgonovo K, Parati MC, Ghilardi M, Amoroso V, Berruti A, Barni S (2019) Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials. Breast Cancer Res Treat 174:597–604. https://doi.org/10.1007/s10549-019-05133-y
Livneh E, Shimon T, Bechor E, Doki Y, Schieren I, Bernard I (1996) Linking protein kinase C to the cell cyele: ectopic expression of PKCll NIH3T3 cells alters the expression of cyelins and Cdk inhibitors and induces adipogenesis. Oncogene 12:1545–1555
Inoue N, Yahagi N, Yamamoto T, Ishikawa M, Watanabe K, Matsuzaka T, Nakagawa Y, Takeuchi Y, Kobayashi K, Takahashi A, Suzuki H, Hasty AH, Toyoshima H, Yamada N, Shimano H (2008) Cyclin-dependent kinase inhibitor, p21WAF1/CIP1, is involved in adipocyte differentiation and hypertrophy, linking to obesity, and insulin resistance. J Biol Chem 283:21220–21229. https://doi.org/10.1074/jbc.M801824200
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67. https://doi.org/10.1016/j.ejca.2015.12.030
Peterson SJ, Mozer M (2017) Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract 32:30–39. https://doi.org/10.1177/0884533616680354
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4:17105. https://doi.org/10.1038/nrdp.2017.105
Acknowledgements
None.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors meet the ICMJE authorship criteria. All authors have seen and approved the final version of the manuscript and contributed significantly to the work. Material preparation, data collection and analysis were performed by KBY, OS, and OY. The first draft of the manuscript was written by KBY, OS and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the institutional ethics committee (date: March 21, 2023, no: 05) and conducted in accordance with the related privacy statements and applicable regulatory requirements.
Consent to participate
This study was conducted in accordance with the provisions of the Declaration of Helsinki and its later amendments. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yücel, K.B., Aydos, U., Sütcüoglu, O. et al. Visceral obesity and sarcopenia as predictors of efficacy and hematological toxicity in patients with metastatic breast cancer treated with CDK 4/6 inhibitors. Cancer Chemother Pharmacol 93, 497–507 (2024). https://doi.org/10.1007/s00280-024-04641-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-024-04641-z